
(R)-1-(4-METHOXYBENZYL)-1 2 3 4 5 6 7 8& synthesis
- Product Name:(R)-1-(4-METHOXYBENZYL)-1 2 3 4 5 6 7 8&
- CAS Number:30356-08-2
- Molecular formula:C17H23NO
- Molecular Weight:257.37
![1,2,3,4,5,6,7,8-octahydro-1-[(4-methoxyphenyl)methyl]isoquinoline](/CAS/GIF/51072-36-7.gif)
51072-36-7
57 suppliers
inquiry

30356-08-2
52 suppliers
inquiry
Yield: 494.5 g
Reaction Conditions:
with (R)-Mandelic Acid in acetone at 25 - 45;Large scale;Reagent/catalyst;
Steps:
4 Example 4
1 kg of the intermediate of formula (I) and 2.8 L of acetone were added to the flask. Stirring at 25 ~ 30°C for 25 to 30 minutes. Then heated to 40 ~ 45°C. Slowly added D-mandelic acid 0.35 kg for chemical resolution. The filtrate was collected after the split; Take one of the filtrate, washed once with 2 L of 10 mol / L sodium hydroxide, 2L water washed twice. The organic phases were combined. Dried over anhydrous sodium sulfate. Filtered and concentrated under reduced pressure to give 1,2,3,4,5,6,7,8-octahydro-R-1-[(4-methoxyphenyl)methyl]isoquinoline formula (III) 494.5 g, HPLC purity 99.7%, the recovery was 98.9%.
References:
Zhejiang Yongtai Pharmaceutical Co., Ltd.;Huang, Jinfeng;Zhou, Guobin;He, Renbao;Zhang, Leigang CN106083717, 2016, A Location in patent:Paragraph 0039; 0040
![3,4,5,6,7,8-hexahydro-1-[(4-methoxyphenyl)methyl]isoquinoline](/CAS/GIF/51072-35-6.gif)
51072-35-6
14 suppliers
inquiry

30356-08-2
52 suppliers
inquiry
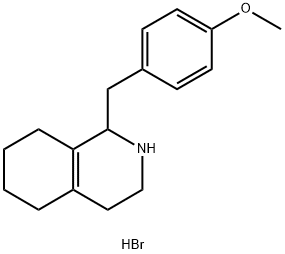
93477-35-1
6 suppliers
inquiry

30356-08-2
52 suppliers
inquiry
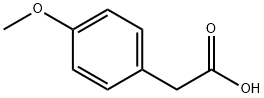
104-01-8
514 suppliers
$6.00/10g
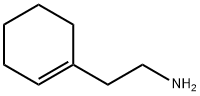
3399-73-3
257 suppliers
$13.00/5g

30356-08-2
52 suppliers
inquiry
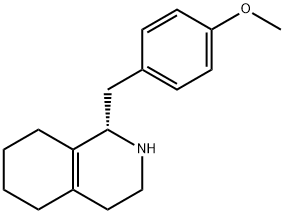
30356-07-1
50 suppliers
inquiry