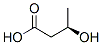
(R)-3-HYDROXYBUTYRIC ACID synthesis
- Product Name:(R)-3-HYDROXYBUTYRIC ACID
- CAS Number:82578-46-9
- Molecular formula:C4H8O3
- Molecular Weight:104.1
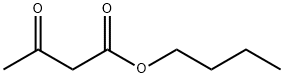
591-60-6
77 suppliers
$80.00/10g
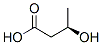
82578-46-9
15 suppliers
$724.92/1G
Yield:93 % ee
Reaction Conditions:
with hydrogen;acetic acid in tetrahydrofuran at 100; under 67506.8 Torr; for 20 h;Autoclave;enantioselective reaction;Temperature;
Steps:
General procedure: Nickel powders (5 μm) purchased from Aldrich were directly subjected to chiral modification without any pretreatment, such as hydrogen activation. The chiral modification was performed under the conditions optimized previously for this type of catalyst.26 Thus, the non-activated nickel powders (0.5 g) were immersed in an aqueous solution (50 cm3) of (R,R)-tartaric acid (0.5g) and NaBr (2.0 g) at 100 °C, the pH of which was pre-adjusted to 3.2 with an aqueous 1M NaOH solution. NaBr was added to the modification solution to block the non-enantiodifferentiating sites of tartaric acid/Ni catalyst, thus preventing the generation of racemic products.39 After immersion for 1 h, the modification solution was removed by decantation and the catalyst was successively washed once with deionized water (10 cm3), twice with methanol (25 cm3), and twice with tetrahydrofuran (THF) (10 cm3). The modified catalyst was added to a mixture of alkyl acetoacetate (43 mmol for methyl ester and 21.5 mmol for other esters), acetic acid (0.1g), and THF (10 cm3) placed in an autoclave equipped with a magnetically coupled mechanical stirrer. The hydrogenation was run for 20h at 100 or 110 °C and at a hydrogen pressure of 9MPa. The hydrogenation product, a mixture of alkyl (R)- and (S)-3-hydroxybutyrates, was isolated from the reaction mixture by distillation. The conversion was determined by gas-liquid chromatography (GLC) on a GL Science model GC-4000 equipped with a CP Chirasil DEX-CB capillary column (0.25 mm × 25 m) at 90 °C, while the enantioselectivity was determined by chiral GLC after acetylation of the reaction product using acetyl chloride and pyridine. A portion of the acetylated sample was subjected to the chiral GLC analysis on a CP Chirasil DEX-CB column (0.25 mm × 25 m) operated at 90 °C. The ee value was calculated from the peak integration of the corresponding enantiomer peaks. The reproducibility of the ee value was found to be within ±2%.
References:
Osawa, Tsutomu;Kizawa, Tomoko;Ikeda, Shinji;Kitamura, Takayuki;Inoue, Yoshihisa;Borovkov, Victor [Tetrahedron Asymmetry,2014,vol. 25,# 24,p. 1630 - 1633]

625-72-9
142 suppliers
$36.00/100mg

71-36-3
1091 suppliers
$14.00/25mL
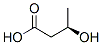
82578-46-9
15 suppliers
$724.92/1G

109-65-9
570 suppliers
$10.00/10g

625-72-9
142 suppliers
$36.00/100mg
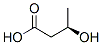
82578-46-9
15 suppliers
$724.92/1G
