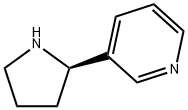
(R)-3-(pyrrolidin-2-yl)pyridine synthesis
- Product Name:(R)-3-(pyrrolidin-2-yl)pyridine
- CAS Number:7076-23-5
- Molecular formula:C9H12N2
- Molecular Weight:148.21
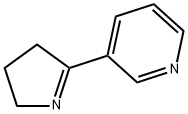
532-12-7
226 suppliers
$35.00/50mg
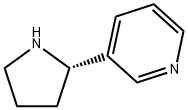
494-97-3
80 suppliers
$109.00/100mg

7076-23-5
45 suppliers
$185.00/100mg
Yield:-
Reaction Conditions:
with NADPHEnzymatic reaction;Reagent/catalyst;
Steps:
1 Example 1
Biotransformations were undertaken at 0.5 ml_ scale with a solution of 10mM myosmine and NADP+ (0.5mM), glucose (25 mM), glucose dehydrogenase (10U/ml), and the enzyme with imine reductase activity. The enzymes used are detailed in table 1 , available from Enzymicals. For each enzyme, the amount of enzyme was 9mg/ml of cell free extract (estimated approx. 0.9mg/ml contained enzyme). For IRED_B and IRED_C specifically, additional tests were run which used 0.9 mg/ml cell free extract. The enantiomeric excess of the (S) nornicotine obtained from the biotransformation was determined using a Chiralpak AD-FI column (250 x 4.6mm id) eluting with a mixture of hexane:ethanol:diethylamine 74.9 : 25.0: 0.1 (v/v/v) at 1 ml/min over 18 min at 30 °C. This method was also used to measure the conversion of myosmine into nornicotine, a relative response factor of 2.18 : 1 having been determined for uv absorption detection at 254 nm. The results are displayed in table 1 below. The % enantiomeric excess for (S)-nornicotine was identified according to the equation [(S)-(R)]/((S)+(R)] c 100 where (S) and (R) are the amounts of (S) and (R) enantiomers present respectively. The % conversion was identified according to the amount of myosmine consumed i.e. according to the equation 100- (final amount of myosmine)/(starting amount of myosmine) * 100.
References:
ZANOPRIMA LIFESCIENCES LIMITED;MCCAGUE, Raymond;NARASIMHAN, Ashok Srinivasan WO2020/98978, 2020, A1 Location in patent:Page/Page column 13
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

7076-23-5
45 suppliers
$185.00/100mg
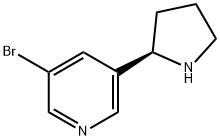
83023-56-7
20 suppliers
inquiry

7076-23-5
45 suppliers
$185.00/100mg
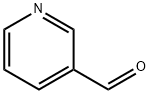
500-22-1
500 suppliers
$6.00/5g

7076-23-5
45 suppliers
$185.00/100mg

20986-40-7
274 suppliers
$6.00/10g

7076-23-5
45 suppliers
$185.00/100mg