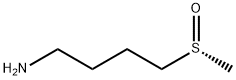
(R)-4-(Methylsulfinyl)-1-butylaMine synthesis
- Product Name:(R)-4-(Methylsulfinyl)-1-butylaMine
- CAS Number:84104-30-3
- Molecular formula:C5H13NOS
- Molecular Weight:135.23

55021-77-7
46 suppliers
$315.00/250mg

84104-30-3
18 suppliers
$496.35/5MG
Yield:84104-30-3 90%
Reaction Conditions:
with dihydrogen peroxide in 2,2,2-trifluoroethanol at 0 - 20; for 1.33333 h;Inert atmosphere;
Steps:
3 EXAMPLE 3 Synthesis of 4-methylsulfinylbutylamine
EXAMPLE 3 Synthesis of 4-methylsulfinylbutylamine [0111] 17.98 g of 4-methylthiobutylamine (1 equivalent) are introduced into the reactor cooled down to 0° C. The assembly is degassed and then placed under nitrogen under stirring. 75 mL of trifluoroethanol are then slowly poured in order to form the amine solution. The addition should be accomplished at 0° C. since exothermy is detected. [0112] 1.1 equivalents of hydrogen peroxide (35% in water) are then poured dropwise into the reactor, within 30 minutes, via an isobaric dropping funnel. [0113] Once the addition is finished, the reaction medium is brought back to room temperature and maintained with stirring, at this temperature, for 1 hour. [0114] This time having elapsed, 3 g of active charcoal are introduced into the reactor. After 20 minutes of stirring at room temperature, the reaction mixture is filtered on celite and the filter is washed with 100 mL of ethanol. [0115] The filtrate is recovered and concentrated in the rotary evaporator at a bath temperature of 50° C. The product is taken up with 60 mL of dichloromethane and dried on MgSO4, and the solvent is then removed with the rotary evaporator. [0116] 18.35 g of a slightly yellow liquid are thereby obtained, i.e. with a yield of 90%. The product is engaged in the following step without requiring any additional purification. [0117] TLC: Eluent: MeOH-5% HCOOH; [0118] Developer: phosphomolybdic acid or ninhydrin; [0119] Rf=0.45. [0120] 1H NMR (300 MHz; CDCl3): [0121] δ (ppm): 2.79-2.62 (m, 4H, -S(O)-CH2-+CH2-NH2); 2.56 (s, 3H, CH3); 1.87-1.76 (m, 2H, -CH2-CH2-); 1.71-1.59 (m, 2H, -CH2-CH2-); 1.57 (broad peak, 2H, NH2). [0122] 1H NMR (300 MHz; D2O): [0123] δ (ppm): 2.92 (m, 2H, -S(O)-CH2-); 2.70 (s, 3H, CH3); 2.68 (t, J=7.0 Hz, 2H, -CH2-NH2); 1.83-1.72 (m, 2H, -CH2-CH2-); 1.67-1.56 (m, 2H, -CH2-CH2-). [0124] 13C NMR (75 MHz; CDCl3): [0125] δ (ppm): 54.39 (-CH2-NH2); 41.56 (-S(O)-CH2-); 38.52 (CH3-); 32.56 (-CH2-CH2-NH2); 19.97 (-S(O)-CH2-CH2-), [0126] 13C NMR (75 MHz; D2O): [0127] δ (ppm): 55.21; 42.81; 39.22; 33.29; 22.29. [0128] Refractive index: nD20=1.4855
References:
US2013/142739,2013,A1 Location in patent:Paragraph 0111-0128
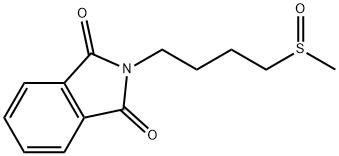
163956-72-7
13 suppliers
inquiry
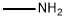
74-89-5
0 suppliers
$13.44/25ML
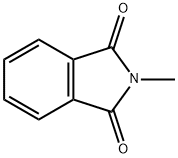
550-44-7
313 suppliers
$20.80/5g

84104-30-3
18 suppliers
$496.35/5MG
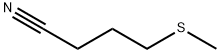
59121-24-3
13 suppliers
inquiry

84104-30-3
18 suppliers
$496.35/5MG

163956-72-7
13 suppliers
inquiry

84104-30-3
18 suppliers
$496.35/5MG
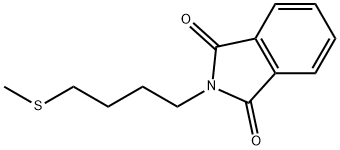
52096-68-1
1 suppliers
inquiry

84104-30-3
18 suppliers
$496.35/5MG