(R)-(+)-N,ALPHA-DIMETHYLBENZYLAMINE synthesis
- Product Name:(R)-(+)-N,ALPHA-DIMETHYLBENZYLAMINE
- CAS Number:5933-40-4
- Molecular formula:C9H13N
- Molecular Weight:135.21
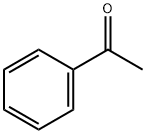
10557-01-4
0 suppliers
inquiry

5933-40-4
89 suppliers
$60.00/50mg
Yield: 94 % ee
Reaction Conditions:
with formic acid;bis[dichloro(pentamethylcyclopentadienyl)iridium(III)];triethylamine;(R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine at 40; for 0.5 h;Inert atmosphere;Flow reactor;
Steps:
Iridium-catalyzed asymmetric reduction of (E)-N-(α-methylbenzylidene)methylamine (20):
To a nitrogen-purged flask was added (E)-N-(α-methylbenzylidene)methylamine (135 mg, 1 mmol), [IrCp*Cl2]2 (4 mg, 0.005 mmol), (R,R)-TsDPEN (4 mg, 0.01 mmol). To this mixture was then added formic acid/triethylamine 5:2 (1 mL). The reaction mixture was stirred under nitrogen at 40 °C for 2 hours. Following this the reaction solution was filtered through silica (1:1 EtOAc/petroleum ether40-60) and the resulting solution dried by rotary evaporation to leave the crude product as a paleyellow oil. The crude was analysed by chiral GC to confirm formation of the product and to determineconversion and enantiomeric excess. GC method: CP-Chirasil-Dex-CB, 25 m 0.25 mm, 0.25 μm. H2 7.5 psi, injector 250 °C, FID 250 °C,oven: 80 °C hold 1 min, 100 °C/min to 100 °C, 13 min hold, 100 °C/min to 200 °C, 5 min hold. (R)-amine 16.6 min. (S)-amine 17.1 min.
References:
Jolley, Katherine E.;Chapman, Michael R.;John Blacker [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2220 - 2228] Location in patent:supporting information
![Carbamic acid, N-[(1R)-1-phenylethyl]-, ethyl ester](/CAS/20200611/GIF/14185-43-4.gif)
14185-43-4
0 suppliers
inquiry

5933-40-4
89 suppliers
$60.00/50mg
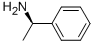
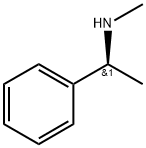
3886-69-9
557 suppliers
$14.00/25G

5933-40-4
89 suppliers
$60.00/50mg

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)