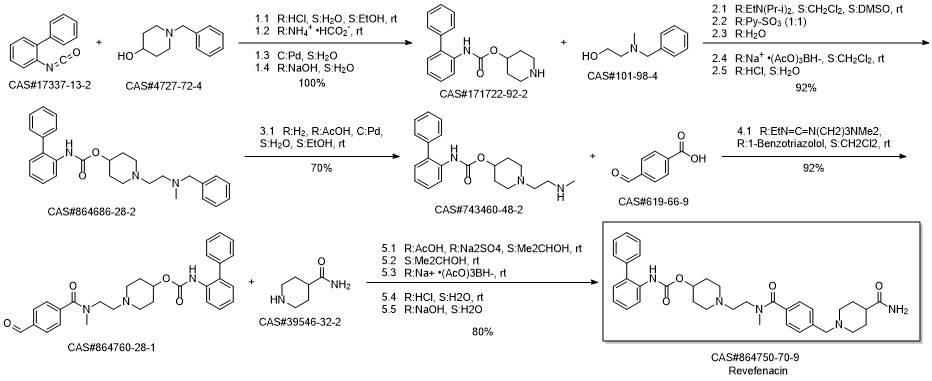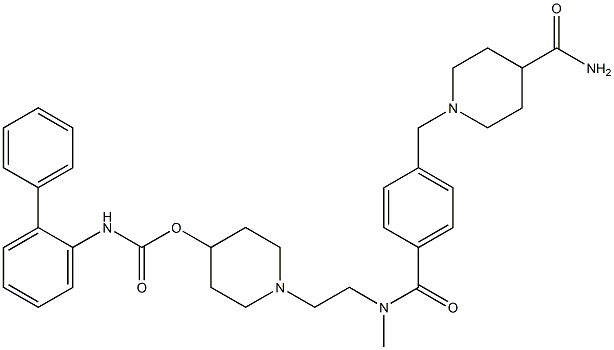
Revefenacin synthesis
- Product Name:Revefenacin
- CAS Number:864750-70-9
- Molecular formula:C35H43N5O4
- Molecular Weight:597.75
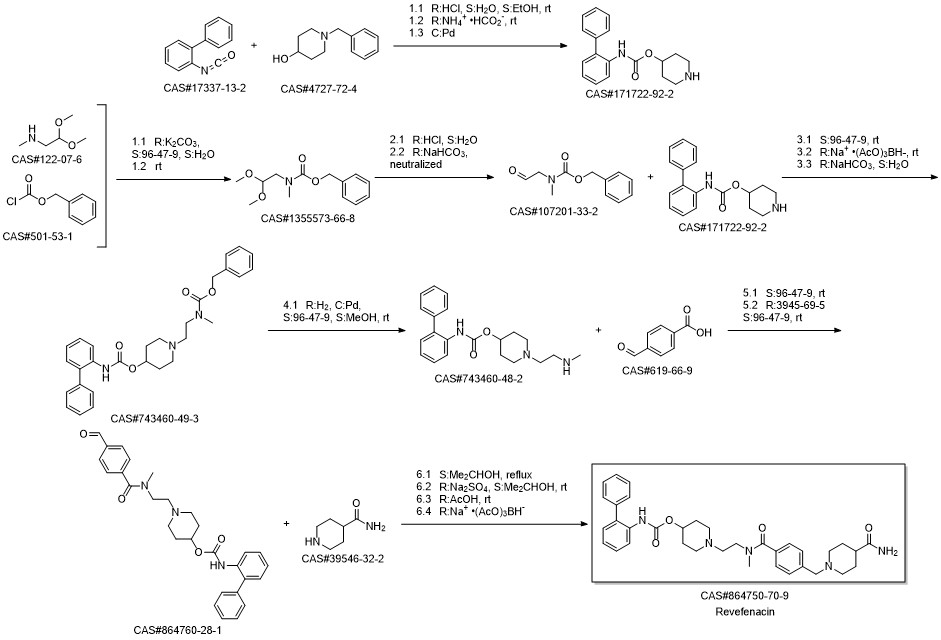
Reference: Colson, Pierre-Jean. Process for preparing a biphenyl-2-ylcarbamic acid 1-(2-{[4-(4-carbamoylpiperidin-1-ylmethyl) benzoyl]methylamino}ethyl)piperidin-4-yl ester. Assignee Theravance, Inc., USA. WO 2012009166. (2012).
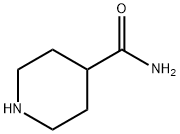
39546-32-2
360 suppliers
$6.00/5g
![1-(2-(3-formyl-N-methylbenzamido)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180702/GIF/864760-28-1.gif)
864760-28-1
30 suppliers
inquiry

864750-70-9
144 suppliers
inquiry
Yield:864750-70-9 85%
Reaction Conditions:
Stage #1: 4-carboxamidopiperidine;biphenyl-2-yl-carbamic acid 1-{2-[(4-formylbenzoyl)methylamino]ethyl}piperidin-4-yl esterwith acetic acid;sodium sulfate in isopropyl alcohol at 0 - 20; for 2 h;
Stage #2: with sodium tris(acetoxy)borohydride in isopropyl alcohol at 0 - 20; for 16 h;Reagent/catalyst;
Steps:
4 Example 4 Synthesis of revefenacin 1
Into a three-necked flask was sequentially added Isonipecotamide 8 (2.30g, 18.0mmol), 5mL of acetic acid,Sodium sulfate 2.50g, isopropanol 10mL.The reaction mixture was cooled to 0-10°C with an ice bath and a solution of IPA (10 mL) containing the product compound 5 (4.32 g, 8.9 mmol) was slowly added.The reaction was stirred at room temperature for 2 hours and then cooled to 0-10°C.NaHB(OAc)3 (5.41 g, 25.5 mmol) was added in portions, and the mixture was stirred at room temperature for 16 hours. Then it was concentrated under reduced pressure to a volume of approximately 50 mL. The mixture was acidified to pH 3 with 1N HCl (200 mL). The resulting mixture was stirred at room temperature for 1 hour, and then extracted with DCM (3*100 mL). Cool the water phase to 0-5°C with an ice bath and add 50% sodium hydroxide aqueous solution to adjust the pH to 10.Then it was extracted with isopropyl acetate (3*100 mL), washed with water (100 mL), brine (2*50 mL) and combined the organic layers,After drying with anhydrous sodium sulfate, filtering and concentrating, it is purified by fast adsorption column.4.54 g of the title compound 1 revefenacin was a white solid substance (HPLC purity: 99.7%, yield 85%).
References:
CN112830890,2021,A Location in patent:Paragraph 0066-0070; 0073; 0080-0081
![piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180702/GIF/171722-92-2.gif)
171722-92-2
103 suppliers
$52.00/250mg

864750-70-9
144 suppliers
inquiry
![benzyl (2-(4-(([1,1'-biphenyl]-2-ylcarbamoyl)oxy)piperidin-1-yl)ethyl)(methyl)carbamate](/CAS/20180703/GIF/743460-49-3.gif)
743460-49-3
9 suppliers
inquiry

864750-70-9
144 suppliers
inquiry