RG-7388 synthesis
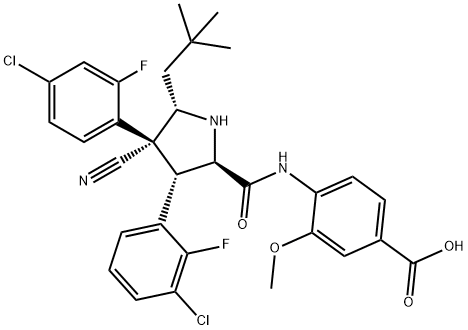
- Product Name:RG-7388
- CAS Number:1229705-06-9
- Molecular formula:C31H29Cl2F2N3O4
- Molecular Weight:616.48

1219086-87-9
15 suppliers
inquiry
![Benzoic acid, 4-[[2-[(E)-(3,3-dimethylbutylidene)amino]acetyl]amino]-3-methoxy-, methyl ester](/CAS/20210305/GIF/1345866-83-2.gif)
1345866-83-2
0 suppliers
inquiry

1229705-06-9
132 suppliers
$41.00/1mg
Yield:1229705-06-9 21%
Reaction Conditions:
Stage #1: (Z)-3-(3-chloro-2-fluorophenyl)-2-(4-chloro-2-fluorophenyl)prop-2-enenitrile;4-{2-[3,3-dimethyl-but-(E)-ylideneamino]acetylamino}-3-methoxybenzoic acid methyl esterwith copper diacetate;(R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl;triethylamine in 2-methyltetrahydrofuran at 20; for 5 h;Inert atmosphere;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;ethanol at 20; for 18 h;Inert atmosphere;
Steps:
1G Example 1G: Synthesis of Idasanutlin (4-((2R,3S,4R,5S)-3-(3-chloro-2- fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-neopentylpyrrolidine-2- carboxamido)-3-methoxybenzoic acid (7))
A solution of CuOAc (0.56 mg, 4.58 pmol) and R- BINAP (3.0 mg, 4.81 miho) in THF (5 mL) was slowly added to a suspension of 3 (302 mg, 0.95 mmol) and 4 (279 mg, 0.90 mmol) in THF (5 ml) under N2 atmosphere at RT. Subsequently, EtaN (123 mL, 0.88 mmol) was added and the resulting mixture was stirred for 5h at RT. Next, THF (10 mL) was added and the resulting solution was washed twice with aq. NH4OAc (10 mL, 10% w/w) and brine (10 mL). Subsequently, the organic layers were evaporated and the crude product was dissolved in THF (7 mL) and EtOH (3 mL). 2.5M aq. NaOH (1 mL) was added and the mixture was stirred for 18h at RT. The solution was acidified with AcOH to pH = 6.0 and the volatiles were partially evaporated (2 mL). After addition of H20 (10 mL) the precipitate was filtered to give the crude product (493 mg, 89%) as an off-white solid. Subsequent enantio-enrichment and purification was performed by crystallization. The crude product (493 mg) was dissolved in THF (6 mL) and heated to 65 °C. Subsequently EtOAc (2 mL) was added and the resulting solution was heated for 15 min after which it was cooled to RT and filtered. The residue was washed with EtOAc (5 mL) and the filtrate evaporated in vacuo. The crude product was dissolved in MeCN (7 mL) and heated to 80 °C after which it was slowly cooled to 10 °C. The precipitate was filtered yielding the pure product (118 mg, 21%) as a white solid. 1H NMR (400 MHz, DMSO-de): d 12.85 (s, 1H), 10.45 (s, 1H), 8.35 (d, J - 8.8 Hz, 1H), 7.71 (t, J - 7.3 Hz, lH), 7.62 - 7.50 (m, 4H), 7.44 - 7.28 (m, 3H), 4.66 - 4.52 (m, 2H), 4.37 (s, 1H), 3.91-3.85 (m, 4H), 1.63 (dd, J - 14.2, 9.9 Hz, 1H), 1.25 (d, J = 14.2 Hz, 1H), 0.96 (s, 9H). HR-MS (ESI, [M+H]+): Calcd. for C31H31CI2F2N3O4: 616.1576; Found: 616.1575
References:
WO2020/71911,2020,A1 Location in patent:Page/Page column 19; 24-25
![Benzoic acid, 4-[[[(2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-diMethylpropyl)-2-pyrrolidinyl]carbonyl]aMino]-3-Methoxy-, Methyl ester](/CAS/20150408/GIF/1229705-05-8.gif)
1229705-05-8
1 suppliers
inquiry

1229705-06-9
132 suppliers
$41.00/1mg
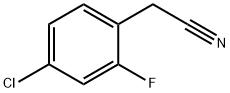
75279-53-7
124 suppliers
$16.00/5g

1229705-06-9
132 suppliers
$41.00/1mg
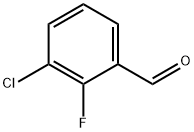
85070-48-0
253 suppliers
$10.00/5g

1229705-06-9
132 suppliers
$41.00/1mg

1219086-87-9
15 suppliers
inquiry

1229705-06-9
132 suppliers
$41.00/1mg