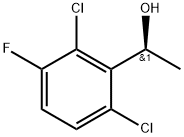
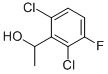
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol synthesis
- Product Name:(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
- CAS Number:877397-65-4
- Molecular formula:C8H7Cl2FO
- Molecular Weight:209.04
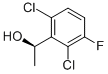
Sodium methoxide (19 mmol; 0.5 M in methanol) was added slowly to compound S-2 (4.64 g, 18.8 mmol) under a nitrogen atmosphere at 0° C. The resulting mixture was stirred at room temperature for 4 hours. The solvent was evaporated and H2O (100 mL) was added. The cooled reaction mixture was neutralized with sodium acetate-acetic acid buffer solution to pH 7. Ethyl acetate (100 mL.x.2) was added to extract the aqueous solution. The combined organic layers were dried over Na2SO4, filtered, and evaporated to obtain S-1 as a white solid (4.36 g, 94.9percent yield); SFC-MS: 97percent ee. 1H NMR (400 MHz, chloroform-D) δ ppm 1.65 (d, J=6.8 Hz, 3 H) 5.58 (q, J=6.9 Hz, 1H) 6.96-7.10 (m, 1H) 7.22-7.36 (m, 1H).

290835-85-7
267 suppliers
$6.00/10g

877397-65-4
279 suppliers
$17.39/250mg
Yield:877397-65-4 100%
Reaction Conditions:
with RuBr2[(S,S)-2,4-bis(diphenylphosphino)pentane](2-aminomethyl-3,5-dimethylpyridine);potassium tert-butylate;hydrogen in isopropyl alcohol at 40; for 21 h;Autoclave;Inert atmosphere;Reagent/catalyst;Concentration;
Steps:
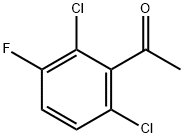
7 The hydrogenating reaction of 2′,6′-dichloro-3′-fluoroacetophenone
In an autoclave, 3.22 mg of RuBr2[(S,S)-xylskewphos](3,5-Me2pica) (3.39×10-3 mmol, S/C=1000) and 7.62 mg of potassium tert-butoxide (6.79×10-2 mmol) are placed, replaced with argon gas. Under argon gas flow, 0.5 mL of 2′,6′-dichloro-3′-fluoroacetophenone (3.39 mmol, from Jiangxi Jixiang Pharmachemical) and 2.9 mL of 2-propanol are added while measuring with syringe, pressurized with hydrogen to 10 atm., stirred at 40° C. for 21 hours, and the reduction of hydrogen pressure was confirmed and (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol was obtained at 100% yield. The optical purity measured by HPLC (DAICEL CHRALPAK AD-RH, acetonitrile/water=25/75, 0.5 mL/min, 25° C., 220 nm, the retention time of each enantiomer: (S): 56.1 min, (R): 64.5 min) was 98.5% ee.
References:
US2015/31920,2015,A1 Location in patent:Paragraph 0168
877397-68-7
4 suppliers
inquiry

877397-65-4
279 suppliers
$17.39/250mg

290835-85-7
267 suppliers
$6.00/10g

877397-65-4
279 suppliers
$17.39/250mg
330156-50-8
180 suppliers
$8.00/1g
756520-66-8
90 suppliers
$10.00/1g

877397-65-4
279 suppliers
$17.39/250mg

877397-67-6
5 suppliers
inquiry

877397-65-4
279 suppliers
$17.39/250mg