
(S)-1-(5-Bromo-pyridin-2-yl)-ethylamine synthesis
- Product Name:(S)-1-(5-Bromo-pyridin-2-yl)-ethylamine
- CAS Number:915720-70-6
- Molecular formula:C7H9BrN2
- Molecular Weight:201.06
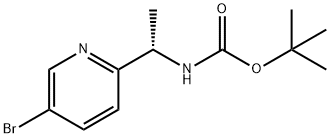
915720-71-7
25 suppliers
$91.00/100mg

915720-70-6
29 suppliers
$338.00/250mg
Yield:915720-70-6 73%
Reaction Conditions:
Stage #1: (S)-tert-butyl (1-(5-bromopyridin-2-yl)ethyl)carbamatewith hydrogenchloride in 1,4-dioxane;dichloromethane at 20; for 1 h;
Stage #2: with sodium hydrogencarbonate in water;
Steps:
26
To a solution of (S)-tert-butyl-l-(5-biOmopyridin-2-yl)ethylcarbamate (Method 27; 2.0 g, 6.6 mmol) in DCM (15 ml) was added HCl/dioxane (17 ml, 4 N, 68 mmol). The reaction was stirred at room temperature for 1 hour. The solvent was removed and 10 ml of saturated sodium bicarbonate was added. The resulting aqueous solution was extracted with ether (6 x 100 ml), dried over sodium sulfate and concentrated to give the title compound (0.98 g, 73%) as a pale yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J= 1.2 Hz, IH), 7.76 (dd, J= 2.0 and 8.4 Hz, IH), 7.24 (d, J= 8.0 Hz, IH), 4.13 (q, J= 6.8 Hz, IH), 1.73 (b, 2H), 1.41 (d, J= 6.4 Hz, 3H).
References:
WO2006/123113,2006,A2 Location in patent:Page/Page column 58
![Acetamide, N-[1-(5-bromo-2-pyridinyl)ethenyl]-](/CAS/20210305/GIF/915720-73-9.gif)
915720-73-9
1 suppliers
inquiry

915720-70-6
29 suppliers
$338.00/250mg
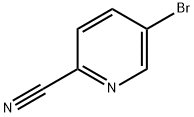
97483-77-7
548 suppliers
$10.00/5g

915720-70-6
29 suppliers
$338.00/250mg
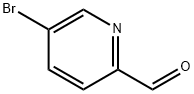
31181-90-5
331 suppliers
$5.00/250mg

915720-70-6
29 suppliers
$338.00/250mg