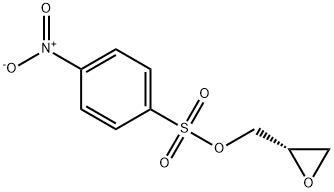
(S)-(+)-Glycidyl-4-nitrobenzenesulfonate synthesis
- Product Name:(S)-(+)-Glycidyl-4-nitrobenzenesulfonate
- CAS Number:118712-60-0
- Molecular formula:C9H9NO6S
- Molecular Weight:259.24
98-74-8
157 suppliers
$10.00/5g

57044-25-4
249 suppliers
$6.00/1g

118712-60-0
86 suppliers
$24.00/250mg
Yield:118712-60-0 40%
Reaction Conditions:
with triethylamine in toluene at 0 - 20; for 0.5 h;
Steps:
S-Oxiran-2-ylmethyl4-nitrobenzenesulfonate (37)
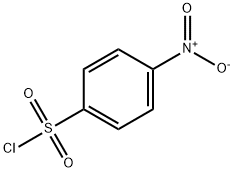
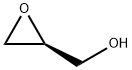
4-Nitrobenzenesulfonylchloride (3.16 g, 14 mmol) was added portionwise to Et3N (2.3 mL,1.67 g, 16 mmol) and R-oxiranylmethanol36 (1.00 g, 14 mmol) in toluene at 0°C. The mixture was stirred at 20°C for 30 min. The suspensionwas filtered (Celite). The evaporation residue, in CH2Cl2,was washed (aq. H2SO4 (2%), sat. aq. NaHCO3,brine). Drying, evaporation and recrystallisation (toluene / hexane) gave 37(1.69 g, 40%) as a white solid: mp 82-83C (lit.5 mp 84-86°C); [a]18D (c = 6.5, CHCl3)+ 33.3 (lit.6 [a]25D+ 26.5° (c 2.45, CHCl3, 82% e.e.)); 1H NMR d 2.60 (1 H, dd, J = 4.7, 2.5 Hz, 3-H), 2.83 (1 H, t, J = 4.4 Hz, 3-H), 3.20 (1 H, m, 2-H), 4.02 (1 H, dd, J = 11.6, 6.4 Hz, SOCH), 4.46 (1 H, dd, J = 11.6, 2.9 Hz, SOCH), 8.12 (2 H, m,Ph 2,6-H2), 8.40 (2 H, m, Ph 3,5-H2); 13C NMR(CDCl3) d 44.42 (3-C), 48.61 (2-C), 71.62 (SOCH2),124.43 (Ph 3,5-C2), 129.24 (Ph 2,6-C2), 141.59 (Ph 1-C),150.84 (Ph 4-C)
References:
Twum, Elvis A.;Nathubhai, Amit;Wood, Pauline J.;Lloyd, Matthew D.;Thompson, Andrew S.;Threadgill, Michael D. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12246,p. 3481 - 3489] Location in patent:supporting information

98-74-8
157 suppliers
$10.00/5g
60456-23-7
242 suppliers
$8.00/1g

118712-60-0
86 suppliers
$24.00/250mg

123750-60-7
74 suppliers
$21.00/1g