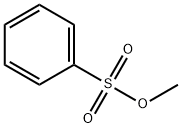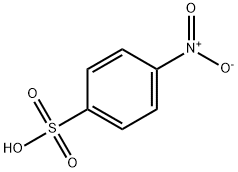
METHYL 4-NITROBENZENESULFONATE synthesis
- Product Name:METHYL 4-NITROBENZENESULFONATE
- CAS Number:6214-20-6
- Molecular formula:C7H7NO5S
- Molecular Weight:217.2
Yield: 88% , 9%
Reaction Conditions:
with Iron(III) nitrate nonahydrate;phosphorus pentoxide in neat (no solvent) at 20; for 6 h;Milling;Green chemistry;Overall yield = 97 percent;
Steps:
General procedure for Table 2 and Table 3
General procedure: A stainless milling beaker of 2.5 mL along with one stainless milling ball ( = 6.0 mm)was used for the reaction. The mechanochemical reaction was performed in a MM400mixer mill at room temperature. A mixture of arene (0.2 mmol), Fe(NO3)3·9H2O (202mg, 0.5 mmol), and P2O5 (213 mg, 1.5 mmol) [For nitrobenzene (Table 2, entry 8), theP2O5 was kept as 284 mg (20 mmol); For methyl benzenesulfonate (Table 2, entry 9),the P2O5 was kept as 426 mg (30 mmol)] were added to the stainless milling beaker,along with one stainless milling ball. The beaker was sealed and placed in the mixermill. The reaction was carried out at 28 Hz for 6 hours. Subsequently, the reactionmixture was dissolved in dichloromethane (15 mL). The resulting mixture was filteredto remove the undissolved residue, and then the solvent was removed via rot-vap undervacuum followed by purification through column chromatography to afford the product.The product was dissolved in a proper deuterium reagent with 7 μl of dibromomethane.The components were analyzed and determined by 1H NMR and literature.
References:
Guo, Zhi-Xin;Wu, Jian-Wei;Zhang, Pu [Tetrahedron Letters,2021,vol. 72,art. no. 153087] Location in patent:supporting information