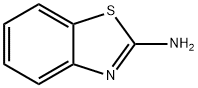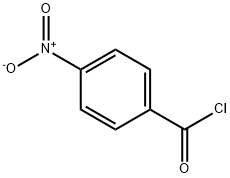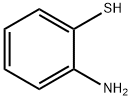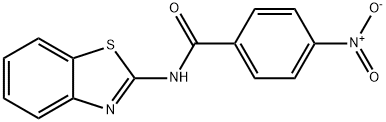
SALOR-INT L449318-1EA synthesis
- Product Name:SALOR-INT L449318-1EA
- CAS Number:35353-21-0
- Molecular formula:C14H9N3O3S
- Molecular Weight:299.3
Yield:35353-21-0 56%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
General procedure: Triethylamine (2 mmol) was added to the solution of aniline derivatives(1 mmol) in anhydrous THF and the appropriate acyl chloride(1.1-2 mmol) was added at 0 °C. The resulting mixture was stirred at room temperature under nitrogen atmosphere for 3-12 h, the eluent was evaporated in vacuo, the residue was partitioned between EtOAc and 2 M NaOH solution. The combined organic layers were washed with NH4Cl ss, brine, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by chromatography on silica gel (eluent: Hexane/ EtOAc 8:2 → 6:4 v/v).
References:
Agamennone, Mariangela;Caradonna, Alessia;Di Pizio, Antonella;Laghezza, Antonio;Loiodice, Fulvio;Luisi, Grazia;Piemontese, Luca;Tortorella, Paolo [Bioorganic and medicinal chemistry,2019]
![2-Benzothiazolamine, N-[(4-nitrophenyl)methylene]-](/CAS/20210305/GIF/61820-61-9.gif)
61820-61-9
0 suppliers
inquiry

35353-21-0
4 suppliers
$28.60/50MG
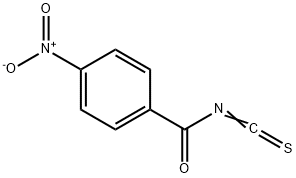
28115-92-6
45 suppliers
$17.00/1g

35353-21-0
4 suppliers
$28.60/50MG