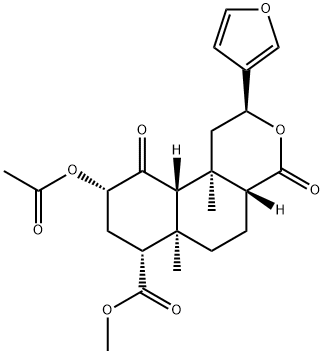
SALVINORIN A synthesis
- Product Name:SALVINORIN A
- CAS Number:83729-01-5
- Molecular formula:C23H28O8
- Molecular Weight:432.46

108-24-7
5 suppliers
$14.00/250ML
92545-30-7
36 suppliers
$29.00/1mg

83729-01-5
38 suppliers
$65.00/1mg
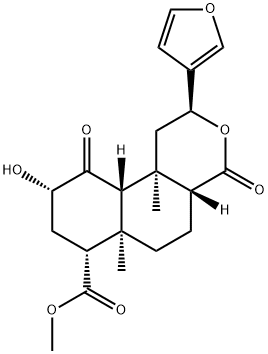
Yield:83729-01-5 0.5%
Reaction Conditions:
with dmap in dichloromethane at 20;
Steps:
1
Dried Salvia divinorum leaves (1.5 kg), obtained commercially from Ethnogens.com, were ground to a fine powder and percolated with acetone (5 x EPO
References:
UNIVERSITY OF IOWA RESEARCH FOUNDATION;BOHN, Laura, M.;PRISINZANO, Thomas, E. WO2006/138589, 2006, A2 Location in patent:Page/Page column 33-34