
SB 223412 synthesis
- Product Name:SB 223412
- CAS Number:174636-32-9
- Molecular formula:C25H22N2O2
- Molecular Weight:382.45
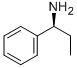
3789-59-1
166 suppliers
$39.06/1g

174636-32-9
82 suppliers
$71.00/100mg
Yield:174636-32-9 79.9%
Reaction Conditions:
Stage #1: oxycinchophenwith triethylamine;1,1'-carbonyldiimidazole in acetonitrile at 20 - 50; for 2 - 5 h;
Stage #2: (R)-1-phenylpropylamine in acetonitrile at 25 - 75;Product distribution / selectivity;
Steps:
1; 2; 3; 4; 5; 6; 7
Example 1; To a 1000 mL 3-neckθd round bottom flask equipped with air-driven mechanical stirrer, thermometer, reflux condenser, addition funnel and nitrogen inlet/outlet, were added 3-hydroxy-2-phenyl-4-quinolinecarboxylic acid (60.0 g, 0.226 mol, 1.00 eq), MeCN (240 mL, 4 vol) and triethylamine (41.0 mL, 0.294 mol, 1.30 eq) at room temperature with stirring. The mixture was stirred at room temperature until a solution was observed. Then 1 , 1'-carbonyldiimidazole (40.3 g, 0.249 mol, 1.10 eq) was charged in one portion. The mixture was heated to 25 0C, and held at the same temperature for 5 h. Then S-1-phenylpropylamine (33.6 g, 0.249 mol, 1.10 eq) was charged in one portion at 25 0C. The mixture was heated to 75 0C, held at 750C for 5 h, cooled to room temperature, and stirred at room temperature overnight. At room temperature, glacial acetic acid (180 mL, 3.0 vol) was added in one portion, causing the solution temperature to rise to -380C. The mixture was cooled slowly from 38 0C to 00C over ca. 4.5 h and the suspension was filtered through a filter paper under vacuum. The cake was washed with cold acetonitrile twice (2 x 120 mL, 2 x 2 vol) and de-ionized water once (120 mL, 2 vol) and dried under vacuum at 70 0C overnight to afford 73.4 g of (-)-(S)-N-(α- ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide as a light yellow to off- white solid, in 84.8% yield.; Example 2; To a 1000 mL 3-necked round bottom flask equipped with air-driven mechanical stirrer, thermometer, reflux condenser, addition funnel and nitrogen inlet/outlet, were added 3-hydroxy-2-phenyl-4-quinolinecarboxylic acid (60.0 g, 0.226 mol, 1.00 eq), ACN (240 mL, 4 vol) and triethylamine (41.0 mL, 0.294 mol, 1.30 eq) at room temperature with stirring. The mixture was stirred at room temperature until a solution was observed. Then 1 , 1'-carbonyldiimidazole (40.3 g, 0.249 mol, 1.10 eq) was charged in one portion. The mixture was heated to 500C, and held at the same temperature for 5 h. Then S-1-phenylpropylamine (33.6 g, 0.249 mol, 1.10 eq) was charged in one portion at 50 0C. The mixture was held at 50 0C for 5 h, cooled to room temperature, and stirred at room temperature EPO
References:
WO2007/16609,2007,A2 Location in patent:Page/Page column 20-24
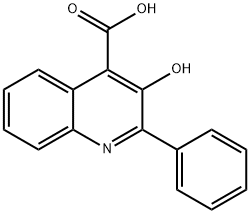
485-89-2
31 suppliers
inquiry

3789-59-1
166 suppliers
$39.06/1g

174636-32-9
82 suppliers
$71.00/100mg
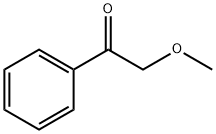
4079-52-1
75 suppliers
$118.00/250mg

174636-32-9
82 suppliers
$71.00/100mg
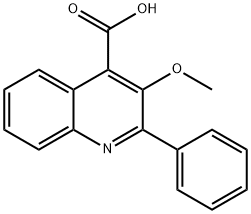
41957-64-6
9 suppliers
inquiry

174636-32-9
82 suppliers
$71.00/100mg

485-89-2
31 suppliers
inquiry

174636-32-9
82 suppliers
$71.00/100mg