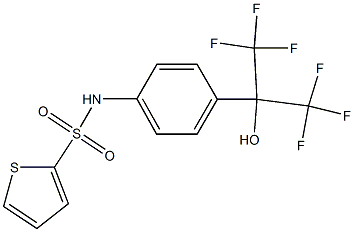
SR3335 synthesis
- Product Name:SR3335
- CAS Number:293753-05-6
- Molecular formula:C13H9F6NO3S2
- Molecular Weight:405.3359

16629-19-9
216 suppliers
$5.00/250mg
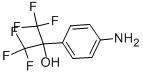
722-92-9
119 suppliers
$8.00/1g

293753-05-6
71 suppliers
$12.00/1mg
Yield:293753-05-6 62%
Reaction Conditions:
with 2,6-dimethylpyridine in acetone at 80; for 24 h;Product distribution / selectivity;Inert atmosphere;
Steps:
A; 7.B
To a solution of 4-(l-hydroxy-l-trifluoromethyl-2,2,2-trifluoroethyl)aniline (A) (1.5M in THF, 128 μ,, 0.193 mmol) in acetone (643 μΙ_) under argon were successively added at room temperature 2,6-lutidine (29 uL, 0.251 mmol) and the corresponding arylsulfonylchloride (0.193 mmol). The mixture was heated at 80°C for 1 day, then cooled to room temperature and diluted with ethyl acetate (EtOAc) and saturated NaHC03 solution. The aqueous phase was extracted two times with EtOAc and combined organic phases were dried over Na2S04, filtrated and evaporated. The crude residue was purified by column chromatography on silica gel to give sulfonamides B. SR3335 was prepared following procedure A and was purified byhexane/EtOAc (7/3) to obtain 48 mg (62%) as a white powder. This compound known in literature and commercially available (CAS 2937-53-05-6).
References:
WO2011/115892,2011,A1 Location in patent:Page/Page column 6; 45; 46
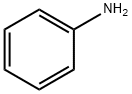
62-53-3
637 suppliers
$10.00/1g

293753-05-6
71 suppliers
$12.00/1mg