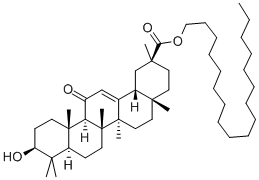
Stearyl glycyrrhetinate synthesis
- Product Name:Stearyl glycyrrhetinate
- CAS Number:13832-70-7
- Molecular formula:C48H82O4
- Molecular Weight:723.16
471-53-4
505 suppliers
$19.80/1G

112-89-0
345 suppliers
$5.00/25g

13832-70-7
177 suppliers
$40.00/5g
Yield:-
Reaction Conditions:
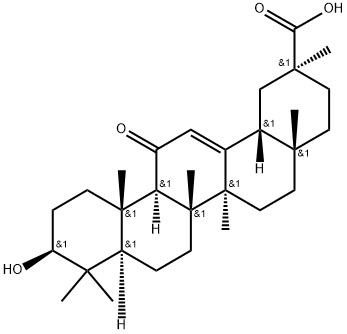
Stage #1:enoxolone with sodium hydroxide in ethanol;water at 50; for 0.5 h;Ionic liquid;
Stage #2:1-Bromooctadecane for 4 h;Reflux;Temperature;
Steps:
1-6 Example 1
Step 1, take 0.7g choline chloride and 0.6g urea, that is, the molar ratio of choline chloride and urea is 1:1.8, addIn a 500mL three-necked flask, place the three-necked flask in a magnetic constant temperature water bath, stir and react for 30min at 80°C, whiteThe solid gradually melts into a clear and transparent liquid, which is a low eutectic ionic liquid;Step 2, dissolve 0.9 g of sodium hydroxide in 1 mL of water to prepare a sodium hydroxide solution, and then combine the sodium hydroxide solution with200mL of ethanol was mixed, added to the low eutectic ionic liquid prepared in step 1, and then 10g of glycyrrhetinic acid, water bath 50React for 30 minutes under C to obtain sodium glycyrrhetinate;Step 3, then add 8g of bromooctadecane to the glycyrrhetinic sodium salt prepared in step 2, and proceed under stirringPerform a water-bath reflux reaction for 4 hours, filter to remove insoluble salts, take the filtrate, cool to room temperature, and filter to obtain stearyl glycyrrhetinateCrude;Step 4, with 150 mL of ethanol on the crude stearyl glycyrrhetinate obtained in step 3 followed by secondary reflux purification30min, filter, and dry under reduced pressure at 50°C to obtain stearyl glycyrrhetinate.
References:
Shanxi Fujie Pharmaceutical Co., Ltd.;Chen Zhenhong;An Chenguang CN111100180, 2020, A Location in patent:Page/Page column 5-6

112-92-5
464 suppliers
$5.00/10g

471-53-4
505 suppliers
$19.80/1G

13832-70-7
177 suppliers
$40.00/5g