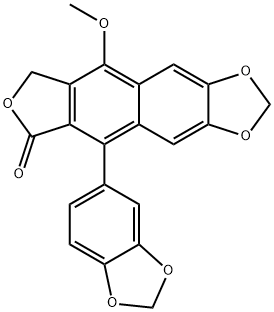
Taiwanin E methyl ether synthesis
- Product Name:Taiwanin E methyl ether
- CAS Number:30403-00-0
- Molecular formula:C21H14O7
- Molecular Weight:378.33
Yield:30403-00-0 78%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 40; for 2 h;
Steps:
13-8 [Example 13-81 Synthesis of5-( 1 ,3-benzodioxol-5-yl)-9-methoxy-furo[3’,4’:6,7 jnaphtho[2,3-dj- 1 ,3-dioxol-6(8H)-on e (justicidin F, taiwanin E methyl ether) [Scheme Fj
6.9 mg of5-( 1 ,3-benzodioxol-5-yl)-9-hydro-furo[3’,4’:6,7 jnaphtho[2,3-dj- 1 ,3-dioxol-6(8H)-one (taiwanin E) (0.019 mmol) was dissolved in 0.19 mL of N,N-dimethylformamide (0.1 M) and 4.0 mg of iodomethane (Mel) (0.028 mmol) and 5.2 mg of potassium carbonate (K2C03) (0.038 mmol) were sequentially added at 0 °C. After increasing reaction temperature to 40 °C, reaction was caffied out for 2 hours. After terminating the reaction by adding 0.5 mL of ammonium chloride aqueous solution, followed by extracting with EtOAc (3 x 1 mL), the organic layer was washed with brine (2 x 1 mL), dried with anhydrous Na2SO4, filtered and then concentrated. The remainder was purified by silica gel column chromatography to obtain 5.6 mg of the target compound 5-( 1 ,3-benzodioxol-5-yl)-9-methoxy-furo[3’,4’:6,7 jnaphtho[2,3-dj- 1 ,3-dioxol-6(8H)-on e (justicidin F, taiwanin E methyl ether) (0.015 mmol, 78%).1H NMR (400 MHz, CDC13) 7.56 (s, 1H), 7.06 (s, 1H), 6.94 (d, 1H, J= 7.6 Hz), 6.77 (dd, 1H, J= 1.2, 4.4 Hz), 6.75 (dd, 1H, J= 1.6, 7.6 Hz), 6.08 (s, 2H), 6.06 (dd, 2H, J= 1.6, 10.0 Hz), 5.51 (s, 2H), 4.09 (s, 3H).
References:
WO2014/119892,2014,A1 Location in patent:Paragraph 121; 378; 379; 380; 381
74879-22-4
1 suppliers
inquiry

30403-00-0
3 suppliers
inquiry
![Naphtho[2,3-d]-1,3-dioxole-6,7-dicarboxylic acid, 5-(1,3-benzodioxol-5-yl)-8-hydroxy-, 6,7-dimethyl ester](/CAS/20210305/GIF/42123-25-1.gif)
42123-25-1
0 suppliers
inquiry

30403-00-0
3 suppliers
inquiry
![5-(1,3-Benzodioxol-5-yl)-9-hydroxyfuro[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(8H)-one](/CAS/GIF/22743-05-1.gif)
