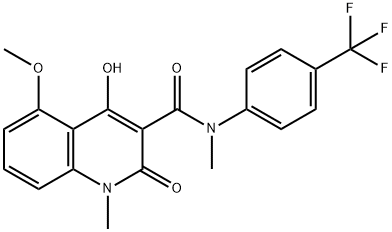
tasquinimod synthesis
- Product Name:tasquinimod
- CAS Number:254964-60-8
- Molecular formula:C20H17F3N2O4
- Molecular Weight:406.36
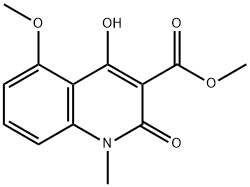
1354639-61-4
14 suppliers
inquiry
22864-65-9
116 suppliers
$14.00/1g

254964-60-8
102 suppliers
$39.00/1mg
Yield: 99%
Reaction Conditions:
in octane for 2 h;Product distribution / selectivity;Reflux;Molecular sieve;
Steps:
4
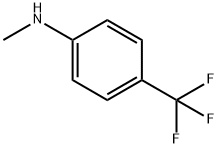
A mixture of 11 (5.00 g, 18.9 mmol), N-methyl-p-trifluoromethylaniline (5.13 g, 28.4 mmol) and n-octane (200 mL) were refluxed in a Soxhlet extraction apparatus containing 4A molecular sieves (22.9 g) for 2 hours. After cooling the mixture the product was isolated as above to furnish 7.6 g (99 %) of 4-hydroxy-5-methoxy-N,l-dimethyl-2-oxo-N-[(4- trifluoromethyl)phenyl] - 1 ,2-dihydroquino line-3 -carboxamide (C) . 1 H-NMR analysis on the isolated product revealed no impurities other than 1 mol% remaining ester 11. 1 H-NMR (CDCI3) 9.9 (s, 1H), 7.50 (bs, 4H), 7.46 (t, 1H), 6.94 (d, 1H), 6.70 (d, 1H), 4.06 (s, 3H), 3.54 (s, 3H), 3.48 (s, 3H). When the same reaction was performed by the traditional distillation from n-octane (Entry 26) during 2 hours according to prior art US patent No. 6,875,869 the product was isolated in 94 % yield and determined by 1H-NMR analysis to consist of a mixture of compound C (96 mol%) and the starting material 11 (4 mol%).
References:
ACTIVE BIOTECH AB;BOCK, Lillemor Maria;HOLMBERG, Pär Henning;JANSSON, Karl-Erik WO2012/4338, 2012, A1 Location in patent:Page/Page column 13; 16

22864-65-9
116 suppliers
$14.00/1g

248282-18-0
10 suppliers
inquiry

254964-60-8
102 suppliers
$39.00/1mg
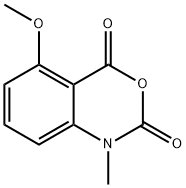
91105-97-4
30 suppliers
$45.00/10mg

254964-60-8
102 suppliers
$39.00/1mg

22864-65-9
116 suppliers
$14.00/1g
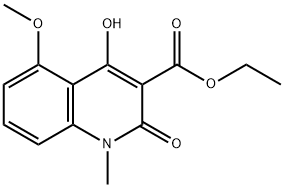
248282-13-5
16 suppliers
inquiry

254964-60-8
102 suppliers
$39.00/1mg

53600-33-2
208 suppliers
$10.00/1g

254964-60-8
102 suppliers
$39.00/1mg