
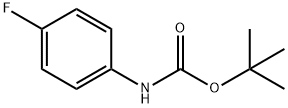
tert-Butyl (2-bromo-4-fluorophenyl)carbamate synthesis
- Product Name:tert-Butyl (2-bromo-4-fluorophenyl)carbamate
- CAS Number:384793-18-4
- Molecular formula:C11H13BrFNO2
- Molecular Weight:290.13

24424-99-5
861 suppliers
$13.50/25G
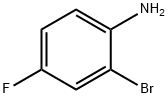
1003-98-1
398 suppliers
$5.00/5g

384793-18-4
14 suppliers
$496.00/1g
Yield:384793-18-4 89%
Reaction Conditions:
with iodine at 20; for 8 h;Inert atmosphere;
Steps:
107 Synthesis of tert-butyl (2-bromo-4-fluorophenyl) carbamate
Synthesis of tert-butyl (2-bromo-4-fluorophenyl) carbamate [0465] To a stirred mixture of 2-bromo-4-fluoroaniline (500 mg, 2.63 mmol) and Boc anhydride (555 mg, 2.76 mmol) under argon atmosphere was added iodine (32 mg, 0.26 mmol) and stirred at RT for 8 h. After consumption of the starting materials (monitored by TLC), the reaction was diluted with water (20 mL) and extracted with EtOAc (2 x 20 mL). The combined organic extracts were washed with a sodium thiosulfate solution (20 mL), dried over sodium sulfate, filtered and concentrated in vacuo. The crude material was purified by column chromatography using 2% EtOAc:hexanes to afford tert-butyl (2-bromo-4- fluorophenyl) carbamate (680 mg, 89%) as colorless oil. 1H-NMR (DMSO-< 5, 400 MHz): δ 8.60 (s, 1H), 7.60 (d, 1H), 7.50-7.45 (m, 1H), 7.23 (t, 1H), 1.43 (s, 9H); LCMS: 289.9 (M+); (column; X-Select CSH C-18 (50 3.0 mm, 3.5 μπι); RT 4.14 min 5mM Aq NH4OAc: ACN; 0.80 mL/min); TLC: 5% EtOAc/hexanes (R 0.6).
References:
WO2015/66697,2015,A1 Location in patent:Paragraph 0465
60144-53-8
51 suppliers
$12.00/1g

384793-18-4
14 suppliers
$496.00/1g

1003-98-1
398 suppliers
$5.00/5g

384793-18-4
14 suppliers
$496.00/1g