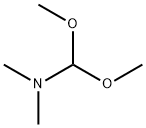
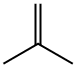
tert-butyl 3-bromo-4-methylbenzoate synthesis
- Product Name:tert-butyl 3-bromo-4-methylbenzoate
- CAS Number:160952-57-8
- Molecular formula:C12H15BrO2
- Molecular Weight:271.15

7697-26-9
266 suppliers
$6.00/5g

160952-57-8
7 suppliers
inquiry
Yield:160952-57-8 95%
Reaction Conditions:
with trifluorormethanesulfonic acid;sodium hydrogencarbonate
Steps:
66.a a)
a) t-butyl 3-bromo-4-methylbenzoate A mixture of 3-bromo-4-methylbenzoic acid (10.75 g, 50 mmol) in dry ether (50 mL) in a glass bomb was cooled to -30° C. and isobutylene gas was bubbled in to give a total reaction volume of approximately 150 mL. Trifluoromethanesulfonic acid (0.22 g, 2.5 mmol) was added dropwise to the stirred mixture, and the bomb was sealed. The reaction was allowed to warm to RT and was stirred for 6 d. The bomb was opened carefully, and 5% sodium bicarbonate (50 mL) was added slowly. The mixture was allowed to stir for 15 min and ether (250 mL) was added. The layers were separated and the organic layer was washed with 5% sodium bicarbonate (2*50 mL) and then with brine (50 mL). Drying (magnesium sulfate) and concentration gave the title compound (12.88 g, 95%) as a yellow oil. TLC Rf 0.68 (toluene); 1H NMR (250 MHz, CDCl3) δ8.13 (d, J=1.7 Hz, 1 H), 7.81 (dd, J=7.9, 1.7 Hz, 1 H), 7.27 (d, J=7.9 Hz, 1 H), 2.44 (s, 3H), 1.59 (s,9 H); IR (CCl4) 1715, 1368, 1297, 1255, 1170, 1123, 1115 cm-1; MS(ES) m/e 273.0 [M+H]+, 271.0 [M+H]+, 214.8 [M+H-C4H8]+.
References:
US6403578,2002,B1

24424-99-5
824 suppliers
$13.50/25G

7697-26-9
266 suppliers
$6.00/5g

160952-57-8
7 suppliers
inquiry