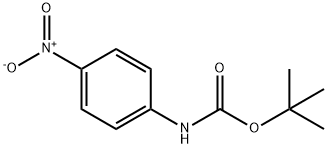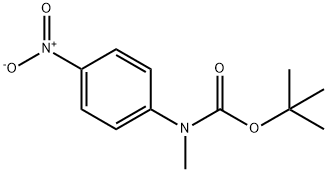
tert-butyl methyl-(4-nitrophenyl)carbamate synthesis
- Product Name:tert-butyl methyl-(4-nitrophenyl)carbamate
- CAS Number:474020-88-7
- Molecular formula:C12H16N2O4
- Molecular Weight:252.27
Yield:474020-88-7 300 mg
Reaction Conditions:
Stage #1: tert-butyl N-(4-nitrophenyl)carbamatewith sodium hydride in tetrahydrofuran;paraffin oil at 0; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran;paraffin oil at 20 - 50; for 16 h;
Steps:
12 Tert-butyl N-methyl-N-(4-nitrophenyl)carbamate
Tert-butyl N-methyl-N-(4-nitrophenyl)carbamate
To a stirred solution of NaH (60% dispersion in paraffin oil) (251 mg, 6.28 mmol) in THF (6.0 mL) at 0° C. was added a solution of tert-butyl N-(4-nitrophenyl)carbamate (1.0 g, 4.19 mmol) in THF (4.0 mL) over a period of 15 min.
The reaction mixture was stirred at this temperature for 15 min and to it was added methyl iodide (590.8 mg, 4.19 mmol).
The reaction mixture was warmed to rt, and was stirred at 50° C. for 16 h.
The mixture was cooled, was quenched with ice cold water (10.0 mL), and was extracted with EtOAc (3*50 mL).
The combined EtOAc extract was washed with water (50 mL), saturated aqueous NaCl (50 mL), dried over Na2SO4 and concentrated and reduced pressure.
The residue was further purified by column chromatography (SiO2, 60-120, hexane/ethylacetete 90/10) to give the title compound (300 mg) as a yellow solid.
References:
US2017/174691,2017,A1 Location in patent:Paragraph 0774; 0775

24424-99-5
840 suppliers
$13.50/25G
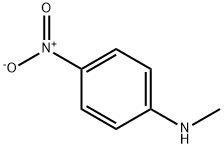
100-15-2
328 suppliers
$10.00/1g

474020-88-7
21 suppliers
inquiry

24424-99-5
840 suppliers
$13.50/25G

100-01-6
22 suppliers
$11.19/25G

474020-88-7
21 suppliers
inquiry

100-01-6
22 suppliers
$11.19/25G

474020-88-7
21 suppliers
inquiry

24424-99-5
840 suppliers
$13.50/25G

474020-88-7
21 suppliers
inquiry