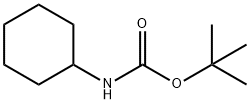
tert-Butyl N-cyclohexylcarbaMate synthesis
- Product Name:tert-Butyl N-cyclohexylcarbaMate
- CAS Number:3712-40-1
- Molecular formula:C11H21NO2
- Molecular Weight:199.29
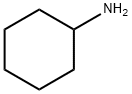
108-91-8
599 suppliers
$10.00/5mL
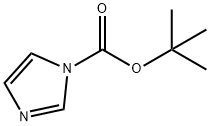
49761-82-2
143 suppliers
$5.00/1g

3712-40-1
13 suppliers
$265.00/2.5g
Yield:3712-40-1 92%
Reaction Conditions:
with Imidazole hydrochloride in water at 20; for 0.166667 h;
Steps:
Synthesis of 1-morpholino-2-phenyl ethanone
General procedure: In a round bottomed flask 0.01 mol (1.36 g) phenyl acetic acid and 0.012 mol (1.94 g) of CDI were added. The reaction mixture was mixed and grinded with a spatula. CO2 gas starts releasing with increase in temperature and solid reaction mixture was turned to pale yellow liquid within 5 min. 0.001 mol (0.1 g) Imidazole. hydrochloride, 0.01 mol (0.87 g) of morpholine, and 1 mL of water were added to it. The reaction mixture was kept at room temperature for another 10 min. Dilute hydrochloride solution was added to it and the aqueous layer was washed with ethyl acetate. The organic layer was dried over anhydrous Na2SO4 and concentrated to give pure product.
References:
Verma, Sanjeev K.;Ghorpade, Ramarao;Pratap, Ajay;Kaushik [Tetrahedron Letters,2012,vol. 53,# 19,p. 2373 - 2376] Location in patent:experimental part

24424-99-5
832 suppliers
$13.50/25G

108-91-8
599 suppliers
$10.00/5mL

3712-40-1
13 suppliers
$265.00/2.5g

24424-99-5
832 suppliers
$13.50/25G
19573-22-9
17 suppliers
inquiry

3712-40-1
13 suppliers
$265.00/2.5g

24424-99-5
832 suppliers
$13.50/25G
87100-15-0
112 suppliers
$9.00/1g

3712-40-1
13 suppliers
$265.00/2.5g
4248-19-5
375 suppliers
$5.00/5g
110-82-7
742 suppliers
$10.00/25g

3712-40-1
13 suppliers
$265.00/2.5g