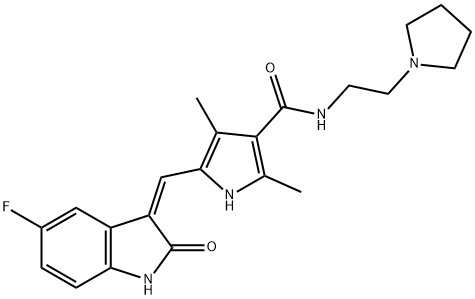
Toceranib synthesis
- Product Name:Toceranib
- CAS Number:356068-94-5
- Molecular formula:C22H25FN4O2
- Molecular Weight:396.46
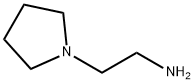
7154-73-6
223 suppliers
$15.00/1g
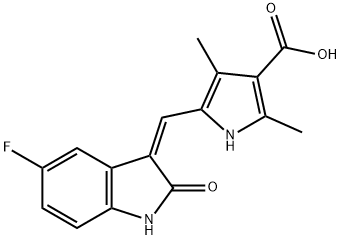
356068-93-4
141 suppliers
$45.00/25mg

356068-94-5
117 suppliers
$28.00/1mg
Yield: 77%
Reaction Conditions:
with (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate;triethylamine in DMF (N,N-dimethyl-formamide) at 20; for 2 h;
Steps:
5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (100 g) and dimethylformamide (500 ml) were stirred and benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (221 g), 1-(2-aminoethyl) pyrrolidine (45.6 g) and triethylamine (93 ml) were added. The mixture was stirred for 2 hours at ambient temperature. The solid product was collected by vacuum filtration and washed with ethanol. The solids were slurry-washed by stirring in ethanol (500 ml) for one hour at 64 C and cooled to room temperature. The solids were collected by vacuum filtration, washed with ethanol, and dried under vacuum to give 5-[5-fluoro-2-oxo-1,2-dihydro-indol- (3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-pyrrolidin-1-yl-ethyl)-amide (101.5 g, 77 % yield). 1H-NMR (dimethylsulfoxide-d6) δ 1.60 (m, 4H, 2xCH2), 2.40, 2.44 (2xs, 6H, 2xCH3), 2.50 (m, 4H, 2xCH2), 2.57, 3.35 (2xm, 4H, 2XCH2), 7.53, 7.70, 7.73, 7.76 (4xm, 4H, aromatic and vinyl), 10.88 (s, 1H, CONH), 13.67 (s, 1H, pyrrole NH). MS m/z 396 [M+1].
References:
PHARMACIA & UPJOHN COMPANY WO2004/76410, 2004, A2 Location in patent:Page 12
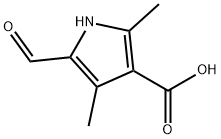
253870-02-9
372 suppliers
$6.00/1g

356068-94-5
117 suppliers
$28.00/1mg

2199-59-9
237 suppliers
$8.00/250mg

356068-94-5
117 suppliers
$28.00/1mg

86770-31-2
148 suppliers
$19.78/1gm:

356068-94-5
117 suppliers
$28.00/1mg
![1H-Pyrrole-3-carboxamide, 5-formyl-2,4-dimethyl-N-[2-(1-pyrrolidinyl)ethyl]-](/CAS/20210111/GIF/342642-56-2.gif)
342642-56-2
0 suppliers
inquiry

56341-41-4
532 suppliers
$12.00/5g

356068-94-5
117 suppliers
$28.00/1mg