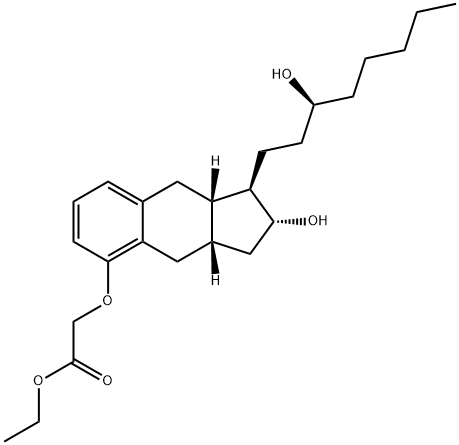
Treprostinil Ethyl Ester synthesis
- Product Name:Treprostinil Ethyl Ester
- CAS Number:1355990-07-6
- Molecular formula:C25H38O5
- Molecular Weight:418.57
Yield:1355990-07-6 96%
Reaction Conditions:
with potassium carbonate;potassium iodide in acetone; for 16 h;Inert atmosphere;Reflux;
Steps:
27 Example 27: ethyl 2-(((l/f,2/f,3a5,9a5)-2-hydroxy-l-((5)-3-hydroxyoctyl)- 2,3,3a,4,9,9a-hexahydro-lH-cyclopenta[b]naphthalen-5-yl)oxy)acetate(25a)
Example 27: ethyl 2-(((l/f,2/f,3a5,9a5)-2-hydroxy-l-((5)-3-hydroxyoctyl)- 2,3,3a,4,9,9a-hexahydro-lH-cyclopenta[b]naphthalen-5-yl)oxy)acetate(25a) [0432] Ethyl bromoacetate was added drop-wise to a slurry of benzindene triol 24a (500 mg, 1.504 mmol, 1.0 equiv), anhydrous potassium carbonate (312 mg, 2.26 mmol, 1.5 equiv) and anhydrous potassium iodide (25 mg, 0.15 mmol, 0.1 equiv) in acetone (20 mL) and the reaction was heated to reflux while stirring, under nitrogen for 16 hours. The reaction was then cooled to room temperature, diluted with heptane (10 mL) and filtered through celite. The celite was rinsed with ethyl acetate (3 x 30 mL) and the filtrate was concentrated to give a pale oil. Chromatography (10% to 80% ethyl acetate/heptane gradient) afforded 610 mg (96%) of the title compound as a colorless oil. Data for 25a: Rf = 0.15 (50% EtOAc / heptane); *H NMR *H NMR (400 MHz, CDC13) δ 7.07 (t, 7=7.87 Hz, 1H), 6.81 (d, 7=7.32 Hz, 1H), 6.64 (d, 7=7.69 Hz, 1H), 4.62 (s, 2H), 4.27 (q, 7=7.32 Hz, 2H), 3.76 (dt, 7=6.04, 9.61 Hz, 1H), 3.55-3.70 (m, 7=4.40 Hz, 1H), 2.89 (dd, 7=5.86, 14.65 Hz, 1H), 2.76 (dd, 7=6.23, 14.28 Hz, 1H), 2.56 (dd, 7=6.59, 15.01 Hz, 1H), 2.46 (dd, 7=6.59, 14.28 Hz, 1H), 2.11-2.34 (m, 4H), 1.89 (tt, 7=6.50, 9.98 Hz, 1H), 1.24-1.75 (m, 16H), 1.12-1.23 (m, 1H), 0.84-0.99 (m, 3H); MS (ESI+) m/z 419.3 (M+H+).
References:
WO2014/89385,2014,A2 Location in patent:Paragraph 0431-0432

64-17-5
707 suppliers
$10.00/50g
81846-19-7
159 suppliers
$95.00/1mg

1355990-07-6
19 suppliers
inquiry
311-28-4
531 suppliers
$5.00/5g

105-36-2
348 suppliers
$5.00/5G
![(1R,2R,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]indene-2,5-diol](/CAS/20180808/GIF/101692-02-8.gif)
101692-02-8
80 suppliers
inquiry

1355990-07-6
19 suppliers
inquiry
40359-32-8
50 suppliers
$31.00/250mg

1355990-07-6
19 suppliers
inquiry
100-83-4
621 suppliers
$5.00/10g

1355990-07-6
19 suppliers
inquiry