REMODULIN synthesis
- Product Name:REMODULIN
- CAS Number:81846-19-7
- Molecular formula:C23H34O5
- Molecular Weight:390.51
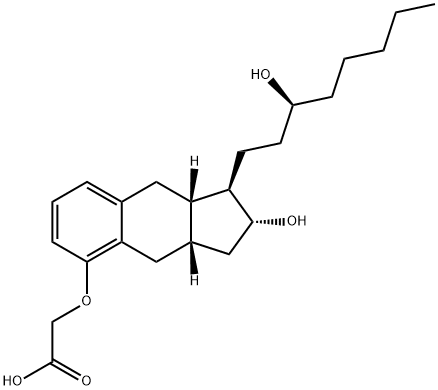
![2-[[(1R,2R,3aS,9aS)-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalen-5-yl]oxy]acetic acid,2-(2-hydroxyethylamino)ethanol](/CAS/20180601/GIF/830354-48-8.gif)
830354-48-8
38 suppliers
$87.00/1mg

81846-19-7
159 suppliers
$79.00/1mg
Yield:81846-19-7 91%
Reaction Conditions:
with hydrogenchloride in water;ethyl acetate; pH=1 for 0.166667 h;Product distribution / selectivity;
Steps:
5
A 250-mL, round-bottom flask equipped with magnetic stirrer was charged with treprostinil diethanolamine salt (4 g) and water (40 mL). The mixture was stirred to obtain a clear solution. To the clear solution, ethyl acetate (100 mL) was added. While stirring, 3M HCl (3.2 mL) was added slowly until pH 1 was attained. The mixture was stirred for 10 minutes and organic layer was separated. The aqueous layer was extracted with ethyl acetate (2×100 mL). The combined organic layers was washed with water (2×100 mL), brine (1×50 mL) and dried over anhydrous Na2SO4. The ethyl acetate solution of treprostinil was filtered and the filtrate was concentrated under vacuum at 50° C. to give off-white solid. The crude treprostinil was recrystallized from 50% ethanol in water (70 mL). The pure treprostinil was collected in a Buchner funnel by filtration and cake was washed with cold 20% ethanolic solution in water. The cake of treprostinil was air-dried overnight and further dried in a vacuum oven at 50° C. under high vacuum to afford 2.9 g of treprostinil (Yield 91.4%, purity (HPLC, AUC, 99.8%).Analytical Data on Treprostinil from Treprostinil Diethanolamine Salt (1:1) to Treprostinil Batch No. Yield Purity (HPLC) 1 91.0% 99.8% (AUC) 2 92.0% 99.9% (AUC) 3 93.1% 99.7% (AUC) 4 93.3% 99.7% (AUC) 5 99.0% 99.8% (AUC) 6 94.6% 99.8% (AUC)
References:
UNITED THERAPEUTICS CORPORATION US2009/163738, 2009, A1 Location in patent:Page/Page column 7-8

101692-03-9
8 suppliers
inquiry

81846-19-7
159 suppliers
$79.00/1mg
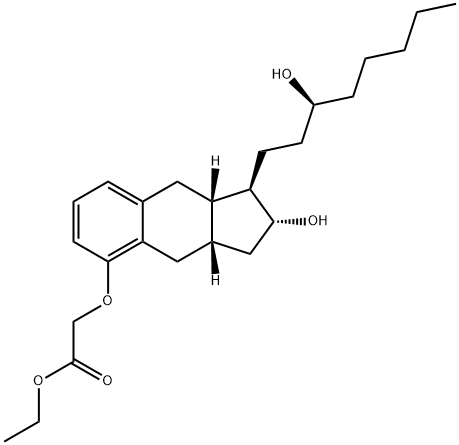
1355990-07-6
19 suppliers
inquiry

81846-19-7
159 suppliers
$79.00/1mg

81845-98-9
11 suppliers
inquiry

81846-19-7
159 suppliers
$79.00/1mg
![(1R,2R,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]indene-2,5-diol](/CAS/20180808/GIF/101692-02-8.gif)
101692-02-8
80 suppliers
inquiry

81846-19-7
159 suppliers
$79.00/1mg