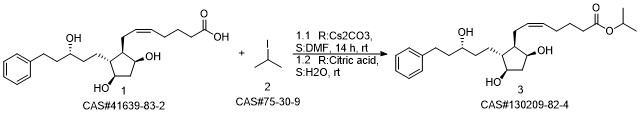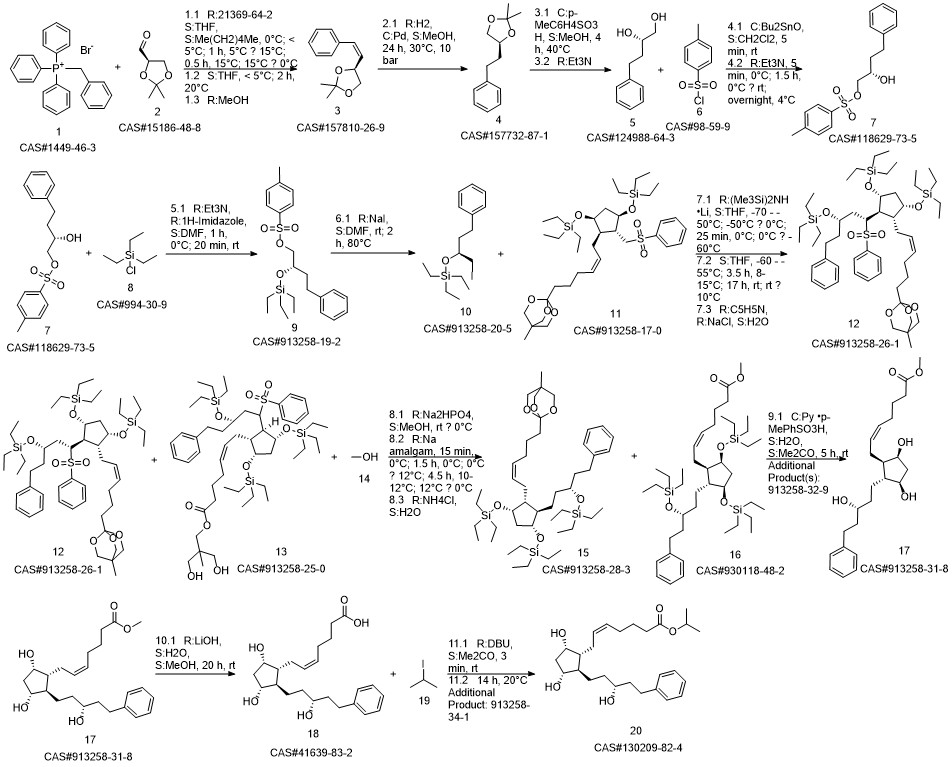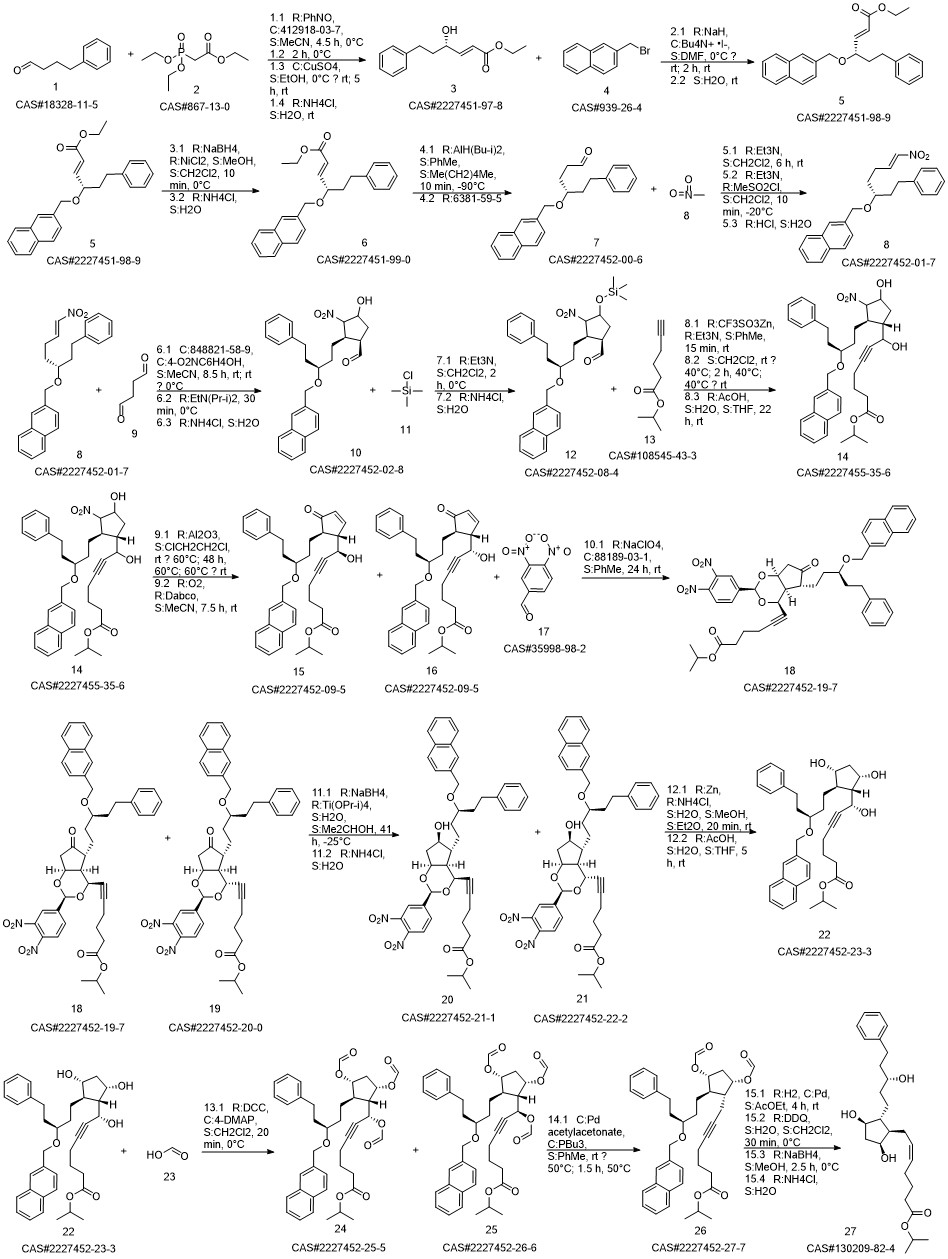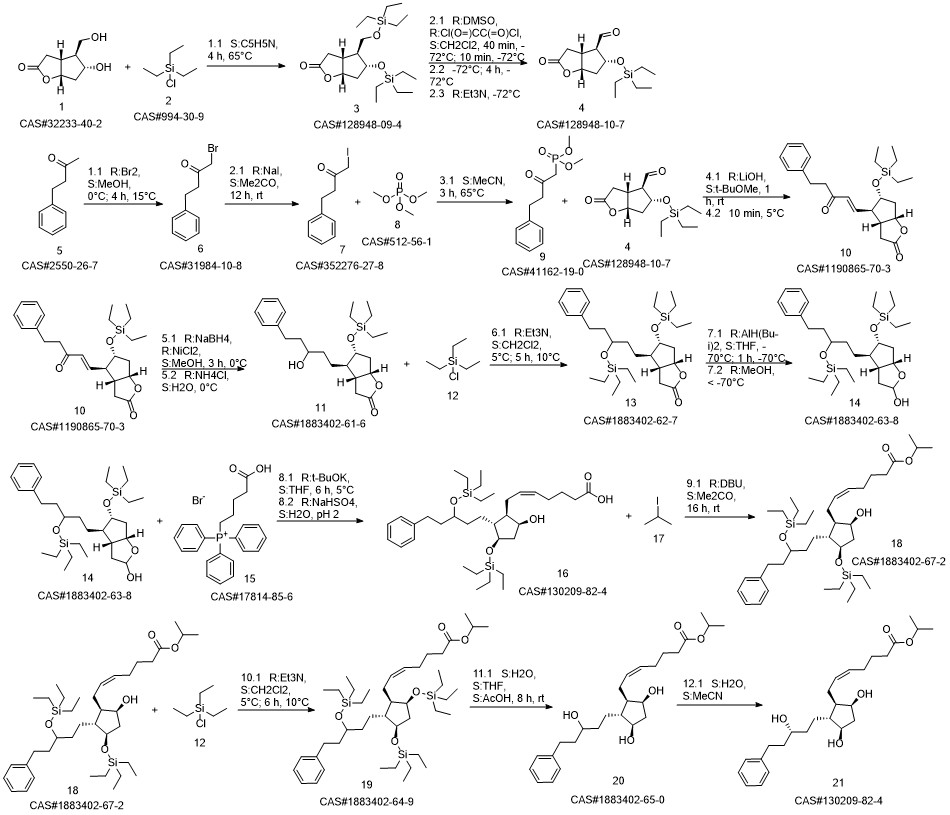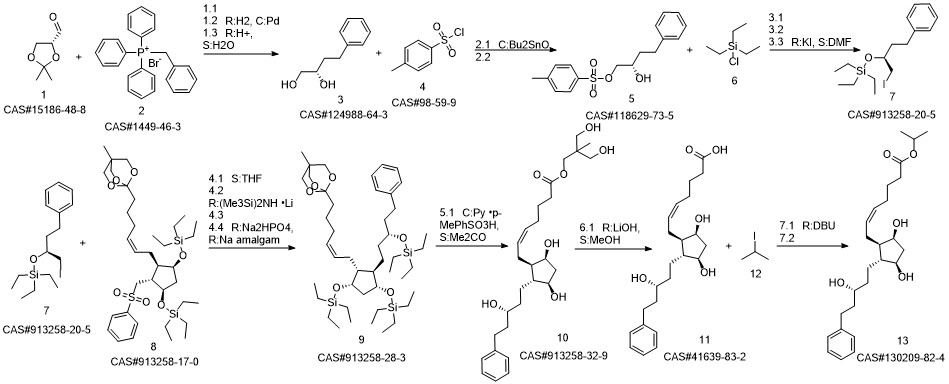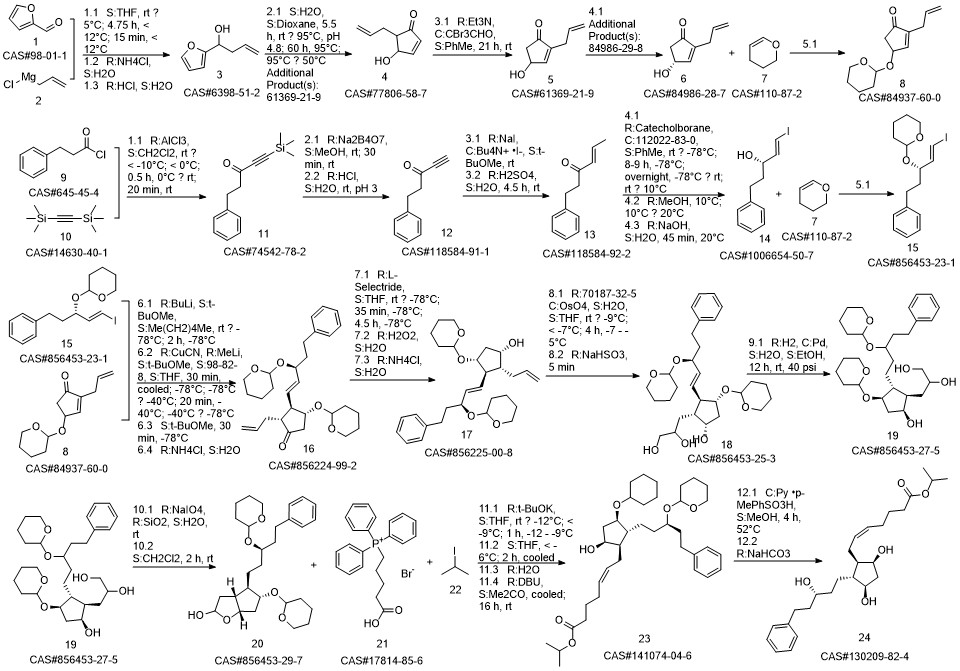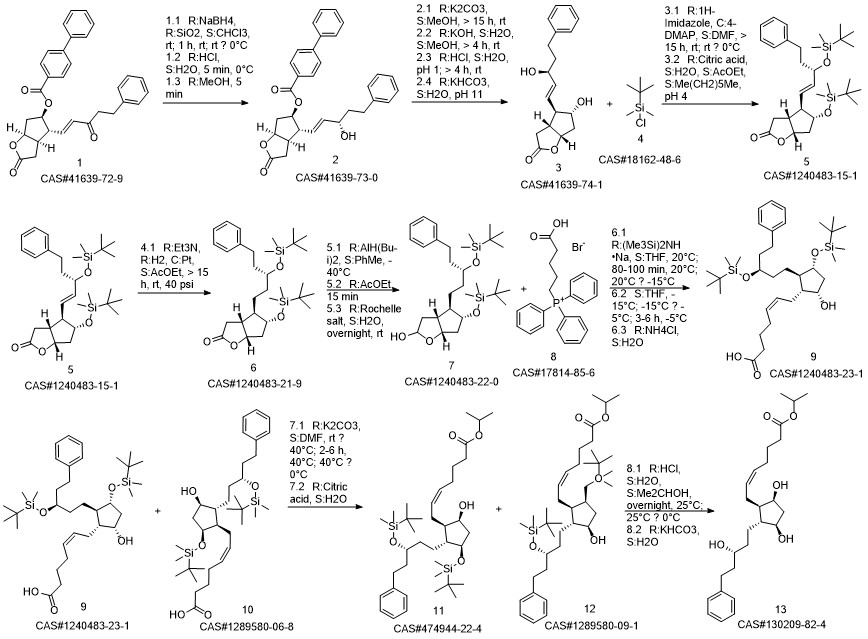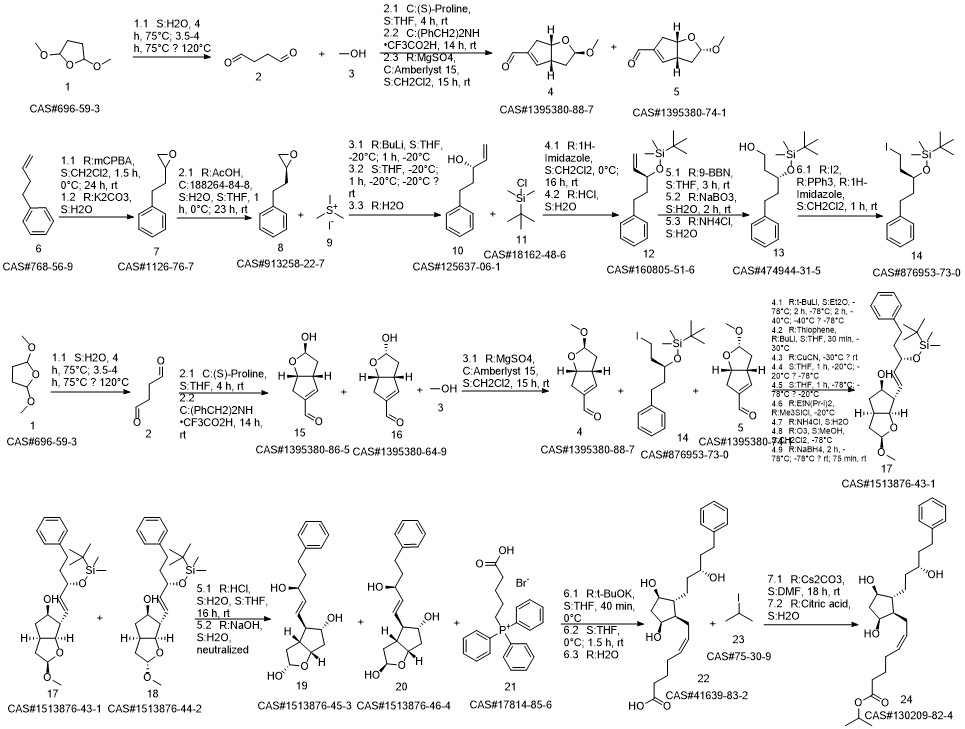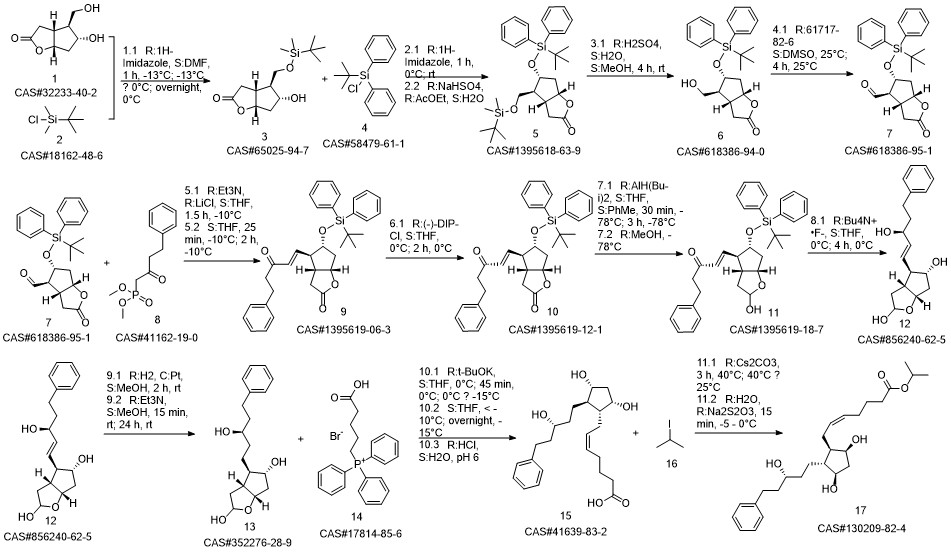
Latanoprost synthesis
- Product Name:Latanoprost
- CAS Number:130209-82-4
- Molecular formula:C26H40O5
- Molecular Weight:432.59
Reference: Prévost S, Thai K, Schützenmeister N, Coulthard G, Erb W, Aggarwal VK. Synthesis of prostaglandin analogues, latanoprost and bimatoprost, using organocatalysis via a key bicyclic enal intermediate. Org Lett. 2015 Feb 6;17(3):504-7. doi: 10.1021/ol503520f. Epub 2015 Jan 12. PubMed PMID: 25582321.
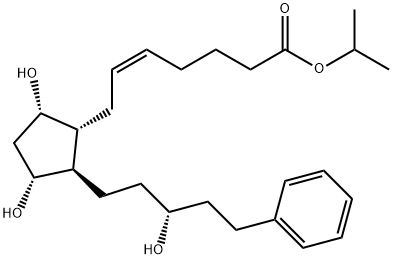
41639-83-2
116 suppliers
$60.00/1mg
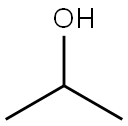
67-63-0
1554 suppliers
$17.00/25ML

130209-82-4
509 suppliers
$50.00/1mg
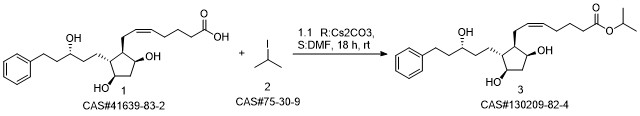
Yield:130209-82-4 92%
Reaction Conditions:
with Lipase Novozym 435 at 30; for 18 h;Enzymatic reaction;
Steps:
4
Preparation of the compound XVI (Latanoprost)The enzyme Lipase Novozym 435 (500 mg) is added to a solution of XV (1 g, 2.56 mmoles) in isopropyl alcohol (10 mL). The solution is kept at 30°C under magnetic stirring (never above 200 rpm). The reaction is complete after 18 hours. The enzyme is simply filtered and recovered, and the solvent is removed at reduced pressure. The product is purified by means of column chromatography (pure AcOEt) to give the pure product in the form of a pale yellow oil with a yield of 92%.
References:
WO2010/97672,2010,A1 Location in patent:Page/Page column 22
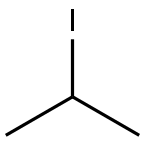
75-30-9
255 suppliers
$15.00/1g

41639-83-2
116 suppliers
$60.00/1mg

130209-82-4
509 suppliers
$50.00/1mg
![5-Heptenoic acid, 7-[(1R,2R,3R)-3-hydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]-5-oxocyclopentyl]-, 1-methylethyl ester, (5Z)-](/CAS/20210305/GIF/141074-06-8.gif)
141074-06-8
1 suppliers
inquiry

130209-82-4
509 suppliers
$50.00/1mg

41639-83-2
116 suppliers
$60.00/1mg

130209-82-4
509 suppliers
$50.00/1mg
![5-Heptenoic acid, 7-[(1R,2R,3R,5S)-2-[(3R)-5-phenyl-3-[(tetrahydro-2H-pyran-2-yl)oxy]pentyl]-3,5-bis[(tetrahydro-2H-pyran-2-yl)oxy]cyclopentyl]-, 1-methylethyl ester, (5Z)-](/CAS/20210305/GIF/856453-33-3.gif)
856453-33-3
0 suppliers
inquiry

130209-82-4
509 suppliers
$50.00/1mg