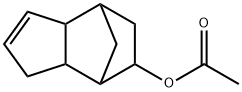
TRICYCLODECENYL ACETATE synthesis
- Product Name:TRICYCLODECENYL ACETATE
- CAS Number:5413-60-5
- Molecular formula:C12H16O2
- Molecular Weight:192.25
Yield:5413-60-5 34%
Reaction Conditions:
at 115 - 140; for 6 h;Product distribution / selectivity;
Steps:
1.B; 2.B; 3; 6.B; 7
B. Synthesis of TCDA. Dicyclopentadiene (“DCPD,” 1048 g, 86% pure) is added dropwise at 115-120° C. to the mixture obtained in Step A over 4 h. The reaction mixture stirs for an additional 2 h at 130° C. and is then cooled to 40° C. Crude material (1564 g) is distilled with aqueous sodium hydroxide (1.0 g of 50% solution) added to the distillation pot. Acetic acid (100 g) is first stripped off at 150 to 1 torr (i.e., 150 to 1 mm Hg). The distillation column is then set for 12 h of total reflux at 1 torr to remove residual acetic acid. Distillation of the remaining material at 1 torr provides commercial-grade TCDA (911 g, 68% based on DCPD). Major isomer by GC: 92.9%; Isomer A: 1.8%; Isomer B: 4.2%; Isomer C, 0.55%.Targeted ranges for TCDA: Major isomer: >90%; Isomer A: <2%; Isomer B: 1-6%; Isomer C: <3%.B. Synthesis of TCDA. DCPD (1048 g, 86% pure) is added dropwise at 115-120° C. to the mixture obtained in Step A over 4 h. The reaction mixture is stirred for an additional 2 h at 130° C. and is then cooled to 40° C. Crude material (1564 g) is distilled with 0.86 g of 50% aqueous NaOH added to the distillation pot. Acetic acid (100 g) is removed at 150-1 torr. The distillation column is then set for 12 h of total reflux at 1 torr to remove residual acetic acid. Distillation of remaining material at 1 torr provides commercial-grade TCDA (947 g, 71% based on DCPD). Major isomer by GC: 92.9%; Isomer A: 1.6%; Isomer B: 4.3%; Isomer C, 0.5%.COMPARATIVE EXAMPLE 3Distillation without Neutralization of Triflic AcidThe procedure of Example 1 is repeated, except that no NaOH solution is added to the distillation pot in Step B. Distillation of the crude reaction mixture (1554 g) provides acetic acid (145 g) and TCDA (670 g, only 47% based on DCPD) that does not meet isomer ratio specifications required for commercial product. Major isomer by GC: 93.1%; Isomer A: 2.5% (too high); Isomer B: 3.0%; Isomer C, 0.67%.B. Synthesis of TCDA. DCPD (1048 g, 86% pure) is added dropwise at 115-140° C. to the mixture obtained in Step A over 3 h. The reaction mixture stirs for an additional 3 h at 140° C. and is then cooled to 40° C. Crude material is distilled with aqueous sodium hydroxide (7.0 g of 50% solution) added to the distillation pot. Acetic acid is first stripped off at 20 torr. The distillation column is then set for 20 h of total reflux at 1 torr to remove residual acetic acid. The remaining material is distilled at 1 torr. The resulting TCDA product (708 g, 73% based on DCPD) meets target specifications regarding isomer content (major isomer by GC: 92.0%; Isomer A: 1.2%; Isomer B: 5.5%; Isomer C, 1.1%). However, the product fails the odor specification because it has a pungent, acidic odor.COMPARATIVE EXAMPLE 7BF3.2H2O CatalystThe procedure of Comparative Example 6 is generally followed, except that 31.0 g of 50% aqueous sodium hydroxide solution (not 7.0 g) is added to the distillation pot in Part B. The resulting TCDA product (441 g, 34% based on DCPD) meets target specifications regarding isomer content (major isomer by GC: 91.7%; Isomer A: 1.4%; Isomer B: 5.6%; Isomer C, 1.1%). However, the product fails the odor specification because it has a pungent, acidic odor.
References:
US7446221,2008,B1 Location in patent:Page/Page column 5; 6; 8
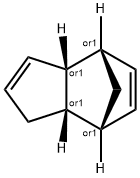
1755-01-7
7 suppliers
inquiry

64-19-7
1628 suppliers
$10.00/25ML

5413-60-5
172 suppliers
inquiry
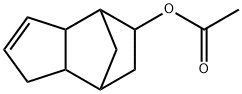
2500-83-6
48 suppliers
inquiry