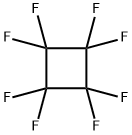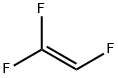
TRIFLUOROETHYLENE synthesis
- Product Name:TRIFLUOROETHYLENE
- CAS Number:359-11-5
- Molecular formula:C2HF3
- Molecular Weight:82.02
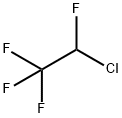
2837-89-0
2 suppliers
inquiry

359-11-5
32 suppliers
$395.00/25 g
Yield:359-11-5 34%
Reaction Conditions:
with methylene chloride;nickel at 650; under 1277.21 Torr;Product distribution / selectivity;
Steps:
14
Process parameters for the process of this invention, employing the preferred Ni-mesh catalyst, were studied in Examples 6 to 14 as described in the following Table 2. TABLE 2 Ex. Temp.CH3ClCF3CFHCl Air % Conversion of % Selectivity toNo.a Catalyst ° C. sccmsccmb SccmCF3CFHClc CF2CHFd 6 Ni-mesh 600 25 50 15 85 16 7 Ni-mesh 650 25 50 15 100 28 8 Ni-mesh 700 25 50 15 100 21 9 Ni-mesh 725 25 50 15 78 14 10 Ni-mesh 650 25 30 15 100 26 11 Ni-mesh 650 25 70 15 94 21 12 Ni-mesh 650 25 102 15 83 14 13 Ni-mesh 650 25 50 10 96 22 14 Ni-mesh 650 25 50 None 100 34f aReaction conditions: pressure, 10 psig; catalyst, 50 cc; bsccm is standard cubic centimeter per minute; cconversion is the ratio of moles of CF3CFHCl reacted to the total moles taken initially multiplied by 100; d% selectivity is the ratio of moles of CF3CFHCl converted to CF2CHF to total moles of CF3CFHCl reacted multiplied by 100; eNo Air is used. fThe yield to trifluoroethylene (R1123) in the absence of air was 34%; however, at this conditions, the major byproduct was 23% of carbon. Table 2 shows results of important process parameter studies. Important parameters such as temperature, flow rate of 1,1,1,2-tetrafluoro-2-chloroethane (R124) and flow rate of air were observed. The reaction is optimized under the conditions given in Table 2, Example No. 7. The highest yield to trifluoroethene (R1123) (34%) was obtained under the conditions given in Table 2, Example No. 14, where the reaction is performed in the absence of air. Although the yield was high, the major problem associated with this condition was the formation of 23% of carbon which eventually killed the catalytic activity after 16 hr of run time. On the contrary, in the presence of air, the catalyst was active for at least 300 hrs. The presence of air is highly desirable to burn out C to CO2 keeping the catalyst surface clean of carbon deposition.;
References:
HONEYWELL INTERNATIONAL INC. US2006/217577, 2006, A1 Location in patent:Page/Page column 4
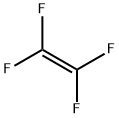
116-14-3
47 suppliers
inquiry

359-11-5
32 suppliers
$395.00/25 g
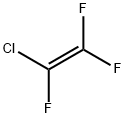
79-38-9
101 suppliers
$270.00/250g

359-11-5
32 suppliers
$395.00/25 g
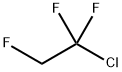
421-04-5
2 suppliers
inquiry

359-11-5
32 suppliers
$395.00/25 g