
Triisopentylamine synthesis
- Product Name:Triisopentylamine
- CAS Number:645-41-0
- Molecular formula:C15H33N
- Molecular Weight:227.43

590-86-3
361 suppliers
$10.00/5g

645-41-0
63 suppliers
$19.80/5g
Yield: 88%
Reaction Conditions:
with 5%-palladium/activated carbon;ammonia;hydrogen in ethanol at 50; under 1125.11 Torr; for 8 h;Solvent;Temperature;Pressure;
Steps:
General procedure: 2.75 g of palladium carbon [5 mass% palladium carbon (56.6 mass% hydrous product, STD type), manufactured by NEC Chemcat Corporation] and 2.75 g of ethanol (Wako Pure Chemical Industries, Ltd.) were placed in a pressure- Manufactured by Kogyo Kabushiki Kaisha) was charged, and the interior of the reactor was replaced three times with a hydrogen pressure of 1 MPa. After returning the pressure inside the reactor to atmospheric pressure, 6 mL (0.24 mol) of liquid ammonia (manufactured by Showa Denko KK) and 80 mL (0.73 mol) of isovaleraldehyde were added in this order, the temperature was raised to 50 ° C., Pressurized to .5 MPa. After stirring at the same temperature and under the same hydrogen pressure for 2 hours, the reactor was cooled to room temperature. Palladium carbon was separated from the reaction mixture by filtration and the resulting reaction mixture was subjected to GC analysis. As a result, the conversion of aldehyde (isovaleraldehyde) was 100%, the yield of tertiary amine (triisoamylamine) was 84%, the yield of secondary amine (diisoamylamine) was 6%, the yield of aldehyde The yield of reduced form (isoamyl alcohol) was 0.4%, and the yield of high boiling substance was 3.8% by mass. The results are shown in Table 1.
References:
Kuraray Co., Ltd.;Shinoda, Osamu JP5658508, 2015, B2 Location in patent:Paragraph 0021; 0029-0030

590-86-3
361 suppliers
$10.00/5g

123-51-3
530 suppliers
$24.00/25mL
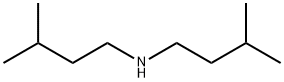
544-00-3
40 suppliers
$116.00/25g

645-41-0
63 suppliers
$19.80/5g

544-00-3
40 suppliers
$116.00/25g

645-41-0
63 suppliers
$19.80/5g

107-85-7
157 suppliers
$10.00/20mg

645-41-0
63 suppliers
$19.80/5g