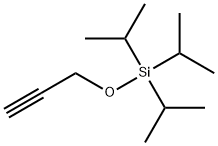
Triisopropyl-prop-2-ynyloxy-silane synthesis
- Product Name:Triisopropyl-prop-2-ynyloxy-silane
- CAS Number:145383-93-3
- Molecular formula:C12H24OSi
- Molecular Weight:212.4
Yield: 95%
Reaction Conditions:
with 1H-imidazole in dichloromethane for 12 h;Inert atmosphere;
Steps:
4.3.2. Preparation of triisopropyl(prop-2-ynyloxy)silane (1)
To a round-bottomed flask under argon were added CH2Cl2 (30 mL), imidazole (1.70 g, 25 mmol), and propargyl alcohol (0.56 g, 0.58 mL, 10 mmol) the mixture was cooled to 0 °C and TIPSCl (2.30 g, 2.55 mL, 12 mmol) was slowly added. The mixture was stirred for 12 h, diluted with CH2Cl2 (20 mL) and quenched with H2O (20 mL). The organic phase was washed with 3% HCl (10 mL), saturated NaHCO3 (20 mL), and finally H2O. The organic phase was then dried over MgSO4, filtered, and concentrated in vacuo. The pure silyl ether was distilled from the residue under reduced pressure (bp 110 °C, 20 mmHg) to yield 2.0 g (95%) of the title compound. 1H NMR (300 MHz, CDCl3) δ 4.37 (d, J=2.4 Hz, 2H, CH2), 2.38 (t, J=2.7 Hz, 1H, CCH), 1.11-1.04 (m, 3H, 3×CH, 18H, 6×CH3); 13C NMR (75 MHz, CDCl3) δ 81.4 (CH×CCH2), 72.5 (CHCCH2), 51.7 (CH2), 17.8 (6×CH3), 11.9 (3×CH).
References:
Oliveira, Juliana M.;R. Freitas, Juliano C.;Comasseto, João Valdir;Menezes, Paulo Henrique [Tetrahedron,2011,vol. 67,# 16,p. 3003 - 3009] Location in patent:experimental part
111998-94-8
0 suppliers
inquiry

145383-93-3
9 suppliers
inquiry

![Methanesulfonimidic acid, 1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]-, tris(1-methylethyl)silyl ester](/CAS/20210111/GIF/213339-58-3.gif)