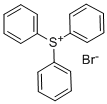
Triphenylsulfonium Bromide synthesis
- Product Name:Triphenylsulfonium Bromide
- CAS Number:3353-89-7
- Molecular formula:C18H15BrS
- Molecular Weight:343.28
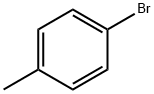
106-38-7
393 suppliers
$14.00/5g

945-51-7
246 suppliers
$5.00/5g

3353-89-7
53 suppliers
$40.00/100mg
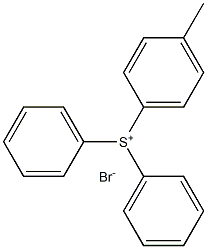
4189-82-6
6 suppliers
inquiry
Yield:4189-82-6 72% ,3353-89-7 1%
Reaction Conditions:
Stage #1: para-bromotoluene;1,1'-sulfinylbisbenzenewith chloro-trimethyl-silane;magnesium in tetrahydrofuran at -5 - 20; for 0.5 h;
Stage #2: with hydrogen bromide in tetrahydrofuran;water;Product distribution / selectivity;
Steps:
2
Comparative Example 1 and Experimental Examples 1 to 6. Effect of the equivalent of an activator relating to the present invention The same procedure as in Example 1 was carried out except for using TMSCl (5 equiv.) used in Example 1 in the various equivalents shown in the following Table 5, to obtain objective 4-methylphenyldiphenylsulfonium bromide. Yields of the obtained objective compound, triphenylsulfonium bromide (byproduct 1) and bis(4-methylphenyl)phenyl sulfonium bromide (byproduct 2) are shown in Table 5.
References:
EP1676835,2006,A1 Location in patent:Page/Page column 21-22

108-86-1
496 suppliers
$10.00/5g

3353-89-7
53 suppliers
$40.00/100mg

108-86-1
496 suppliers
$10.00/5g

945-51-7
246 suppliers
$5.00/5g

3353-89-7
53 suppliers
$40.00/100mg

945-51-7
246 suppliers
$5.00/5g
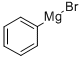
100-58-3
185 suppliers
$18.00/10ml

3353-89-7
53 suppliers
$40.00/100mg

945-51-7
246 suppliers
$5.00/5g
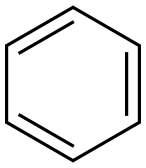
71-43-2
675 suppliers
$11.19/25ML

3353-89-7
53 suppliers
$40.00/100mg