
Triphenylsulfonium chloride synthesis
- Product Name:Triphenylsulfonium chloride
- CAS Number:4270-70-6
- Molecular formula:C18H15ClS
- Molecular Weight:298.83
The water layer was separated and washed with 100 g of diethyl ether, yielding an aqueous solution of triphenylsulfonium chloride. The compound in aqueous solution form was used in the subsequent reaction without further isolation.
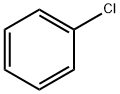
108-90-7
658 suppliers
$10.00/25G

4270-70-6
127 suppliers
$71.00/5g
Yield:4270-70-6 67%
Reaction Conditions:
Stage #1: chlorobenzenewith iodine;magnesium in tetrahydrofuran;ethylene dibromide at 50; for 5 h;Inert atmosphere;
Stage #2: with chloro-trimethyl-silane in tetrahydrofuran;ethylene dibromide; for 2 h;Inert atmosphere;Further stages;Solvent;
Steps:
1.1-1.2-2.1-2.2
S01, under the protection of nitrogen gas, add 4.8g magnesium scraps and 2 iodine particles into the first organic solvent (100ml tetrahydrofuran),Then add dropwise initiator (2ml dibromoethane, 50 , visible bubbles and reflux solution after initiation, indicating that initiation is completed),Then the substrate solution (mixed solution of 100ml of tetrahydrofuran and 20g of chlorobenzene) was added dropwise for the reaction (the dropwise temperature was 50°C, the dropwise time was 3h, and the temperature was kept at 50°C for 2h after the dropwise addition), GC detected chlorobenzene<0.5 % Stop the reaction to obtain the first reaction solution, add an aging solution (40 ml of a tetrahydrofuran solution containing 2.6 g of trimethylchlorosilane) to the first reaction solution, and perform an aging reaction for 2 h to obtain a second reaction solution (GC detection raw material chlorobenzene) less than 5%);S02, under the protection of nitrogen gas, cool the second reaction solution to 0°C, add thionyl chloride solution to the second reaction solution, dropwise at a temperature of 0°C, and add dropwise for 3 hours to obtain a mixed solution; The mixed solution is placed in (5% concentration of hydrogen chloride solution, 30g) hydrogen chloride ice water (every hundred milliliters of hydrogen chloride ice water contains 3g hydrogen chloride), and chlorination is carried out at 10 ° C to obtain a chlorination reaction solution, pH=25; The chlorination reaction solution was filtered, the filtrate was taken, left to separate layers, and then the aqueous layer was taken, washed with the second organic solvent (diethyl ether, 40ml*3) to obtain an aqueous solution of triphenylsulfonium salt; The conversion of triphenylsulfonium salt was 67%.
References:
CN114262286,2022,A Location in patent:Paragraph 0045; 0049-0056

945-51-7
255 suppliers
$5.00/5g
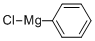
100-59-4
264 suppliers
$42.00/0.25mole

4270-70-6
127 suppliers
$71.00/5g

945-51-7
255 suppliers
$5.00/5g

4270-70-6
127 suppliers
$71.00/5g
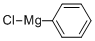
100-59-4
264 suppliers
$42.00/0.25mole

4270-70-6
127 suppliers
$71.00/5g