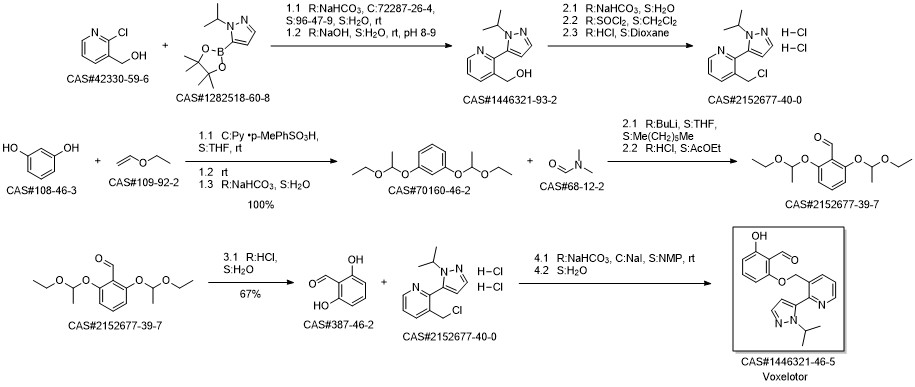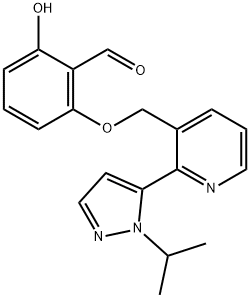
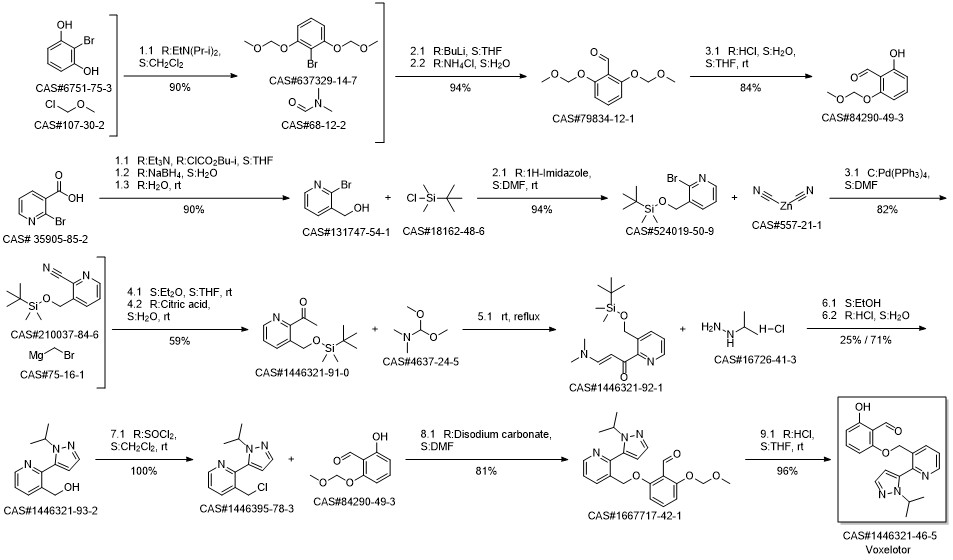
Voxelotor synthesis
- Product Name:Voxelotor
- CAS Number:1446321-46-5
- Molecular formula:C19H19N3O3
- Molecular Weight:337.37
Reference: Metcalf, Brian; Chuang, Chihyuan; Dufu, Kobina; Patel, Mira P.; Silva-Garcia, Abel; Johnson, Carl; Lu, Qing; Partridge, James R.; Patskovska, Larysa; Patskovsky, Yury; Almo, Steven C.; Jacobson, Matthew P.; Hua, Lan; Xu, Qing; Gwaltney, Stephen L.; Yee, Calvin; Harris, Jason, Morgan, Bradley P.; James, Joyce; Xu, Donghong; Hutchaleelaha, Athiwat; Paulvannan, Kumar; Oksenberg, Donna; Li, Zhe. Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin. ACS Medicinal Chemistry Letters. Volume 8. Issue 3. Pages 321-326. Journal; Online Computer File. (2017).
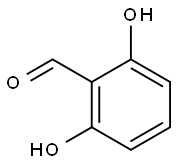
387-46-2
174 suppliers
$38.00/1g

1446321-95-4
27 suppliers
inquiry

1446321-46-5
102 suppliers
$45.00/1mg
Yield:1446321-46-5 88%
Reaction Conditions:
Stage #1:2,6-dihydroxybenzaldehyde with potassium carbonate in N,N-dimethyl-formamide at 20; for 0.166667 h;Inert atmosphere;
Stage #2:3-(chloromethyl)-2-(1-isopropyl-1H-pyrazol-5-yl)pyridine hydrochloride in N,N-dimethyl-formamide at 20 - 50; for 2 h;Inert atmosphere;
Steps:
18 Example 18. Preparation of 2-hydroxy-6-((2-(l-isopropyl-lH-pyrazol-5-yl)pyridin-3- yl)methoxy)benzaldehyde (Compound 43).
[0179] A mixture of 2,6-dihydroxybenzaldehyde (1.58 g, 11.47 mmol, 2 eq.) and K2C03 (2.4 g, 17.22 mmol, 3 eq.) in DMF (150 mL) was stirred at rt for 10 min. To this mixture was added 3-(chloromethyl)-2-(l-isopropyI-lH-pyrazol-5-yl)pyridine hydrochloride (1.56 g, 5.74 mmol, leq.) at rt. The mixture was heated at 50 °C for 2 h, filtered, concentrated and purified on silica gel using a mixture of EtOAc and hexanes as eluent to give 2-hydroxy-6-((2-(l- isopropyl-lH-pyrazol-5-yl)pyridin-3-yl)methoxy)benzaldehyde (1.71 g, 88%) as a pale yellow solid. NMR (400 MHz, CDC13) δ 11.96 (s, 1H), 10.40 (s, 1H), 8.77 (dd, J= 4.8, 1.5 Hz, 1H), 8.00 (d, J= 7.8 Hz, 1H), 7.63 (d, J= 1.8 Hz, 1H), 7.49 - 7.34 (m, 2H), 6.59 (d, J = 8.5 Hz, 1H), 6.37 (d, J= 1.8 Hz, 1H), 6.29 (d, J= 8.2 Hz, 1H), 5.10 (s, 2H), 4.67 (sep, J = 6.7 Hz, 1H), 1.50 (d, J= 6.6 Hz, 6H). LRMS (M+rf") m/z 338.1
References:
GLOBAL BLOOD THERAPEUTICS, INC.;CYTOKINETICS, INC.;THE REGENTS OF THE UNIVERSITY OF CALIFORNIA;METCALF, Brian;CHUANG, Chihyuan;WARRINGTON, Jeffrey;PAULVANNAN, Kumar;JACOBSON, Matthew P.;HUA, Lan;MORGAN, Bradley WO2013/102142, 2013, A1 Location in patent:Paragraph 0179

387-46-2
174 suppliers
$38.00/1g
![[2-[1-(propan-2-yl)-1H-pyrazol-5-yl]pyridin-3-yl]methanol](/CAS/20180629/GIF/1446321-93-2.gif)
1446321-93-2
11 suppliers
inquiry

1446321-46-5
102 suppliers
$45.00/1mg
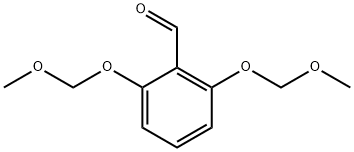
79834-12-1
4 suppliers
inquiry

1446321-46-5
102 suppliers
$45.00/1mg
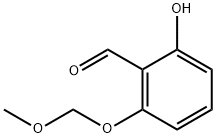
84290-49-3
42 suppliers
inquiry

1446321-46-5
102 suppliers
$45.00/1mg
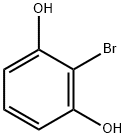
6751-75-3
145 suppliers
$8.00/250mg

1446321-46-5
102 suppliers
$45.00/1mg