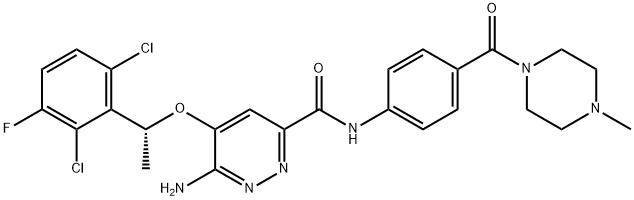
X-376 synthesis
- Product Name:X-376
- CAS Number:1365267-27-1
- Molecular formula:C25H25Cl2FN6O3
- Molecular Weight:547.41
=C1Cl)Cl)OC2=CC(C(NC3=CC=C(C(N4CCN(CC4)C)=O)C=C3)=O)=NN=C2N(C(OC(C)(C)C)=O)C(OC(C)(C)C)=O](/CAS/20211123/GIF/1370651-39-0.gif)
1370651-39-0
1 suppliers
inquiry

1365267-27-1
75 suppliers
$28.00/1mg
Yield:1365267-27-1 80.5%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 2 h;
Steps:
1.2
lb (12.43g, 16.7mmol) was dissolved in a mixture of DCM (90mL) and TFA (30mL), stirred at r.t. for 2 hours and evaporated. The residue was adjusted by sat. Na2C03 to pH=8 and extracted with DCM (150mLx5). The combined organic phase was dried over MgS04 and concentrated. The residue was triturated with methanol and filtered, then the solid was dissolved in DCM and a solution of HC1 in Et20 was added, the mixture was stirred at r.t. overnight, then concentrated and dried over oil pump to afford 1 (8.31g, 80.5%). 1H-NMR (300MHz, DMSO-d6): 5=1.84 (d, 3H), 2.76 (d, 3H), 3.02-3.10 (m, 2H), 3.37-3.53 (m, 5H), 3.40-4.26 (m, 1H), 6.27 (q, 1H), 7.11 (s, IH), 7.42-7.51 (m, 3H), 7.58-7.62 (m, IH), 7.86-7.88 (m, 2H). LC-MS[M+H]+:547.2.
References:
WO2012/48259,2012,A2 Location in patent:Page/Page column 39-40
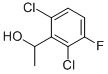
756520-66-8
91 suppliers
$10.00/1g

1365267-27-1
75 suppliers
$28.00/1mg
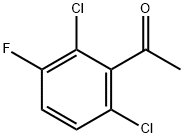
290835-85-7
259 suppliers
$6.00/10g

1365267-27-1
75 suppliers
$28.00/1mg
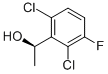
330156-50-8
175 suppliers
$8.00/1g

1365267-27-1
75 suppliers
$28.00/1mg
![3-Pyridazinamine, 6-chloro-4-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-](/CAS/20200611/GIF/1370651-29-8.gif)
1370651-29-8
4 suppliers
inquiry

1365267-27-1
75 suppliers
$28.00/1mg