
(+)-YK-4-279 synthesis
- Product Name:(+)-YK-4-279
- CAS Number:1261038-93-0
- Molecular formula:C17H13Cl2NO4
- Molecular Weight:366.2

18711-13-2
143 suppliers
$7.00/250mg
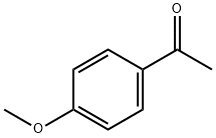
100-06-1
598 suppliers
$18.19/25g

1261038-93-0
2 suppliers
$60.00/1mg
Yield:1261038-93-0 97%
Reaction Conditions:
Stage #1: 4,7-dichloroisatinwith N-[3,5-bis(trifluoromethyl)phenyl]-N'-[(9R)-6'-methoxycinchonan-9-yl]thiourea in tetrahydrofuran at 5; for 0.166667 h;
Stage #2: 1-(4-methoxyphenyl)ethanone in tetrahydrofuran at 5; for 144 h;optical yield given as %ee;enantioselective reaction;
References:
Guo, Qunsheng;Bhanushali, Mayur;Zhao, Cong-Gui [Angewandte Chemie - International Edition,2010,vol. 49,# 49,p. 9460 - 9464] Location in patent:experimental part