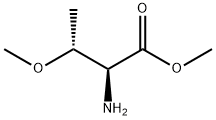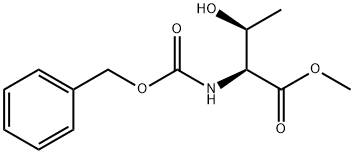
Z-Allo-Thr-OMe synthesis
- Product Name:Z-Allo-Thr-OMe
- CAS Number:98777-34-5
- Molecular formula:C13H17NO5
- Molecular Weight:267.28
Yield:98777-34-5 86%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 0 - 26; for 16.5 h;
Steps:
190 (2S,3S)-Methyl 2-(((benzyloxy)carbonyl)amino)-3-hydroxybutanoate
To a stirred solution of (2S,3S)-methyl 2-amino-3-hydroxybutanoate (2.5 g, 14.73 mmol) in a 1:1 mixture of tetrahydrofuran and water (50 mL), sodium bicarbonate (1.9 g, 22.10 mmol) was added and the mixture was cooled to 0° C. and to this benzyl chloroformate (2.76 g, 16.21 mmol) was added drop wise over a period of 30 minutes. The mixture was then stirred at the room temperature for 16 hours. The reaction mixture was diluted with ethyl acetate (25 mL), organic layers were separated, washed with brine (25 mL) and dried over sodium sulfate. Removal of solvents under vacuum afforded the crude product which has been purified by silica gel column chromatography (230-400 mesh) using a gradient of ethyl acetate in pet.ether. Yield: 3.4 g (86%) 1H NMR (400 MHz, DMSO-d6) δ: 1.09 (d, 3H), 3.89 (s, 3H), 3.99 (t, 1H), 4.01-4.06 (m, 1H), 5.00 (d, 1H), 5.04 (s, 2H), 7.30-7.40 (m, 5H), 7.63 (d, 1H). MS (ES) MH+: 268.1 for C13H17NO5
References:
US2014/206677,2014,A1 Location in patent:Paragraph 0727; 0728
![4H-1,3-Oxazin-4-one, tetrahydro-6-methyl-5-[(4S)-2-oxo-4-phenyl-3-oxazolidinyl]-2-[(1S)-1-[[tris(1-methylethyl)silyl]oxy]ethyl]-, (2R,5R,6S)-](/CAS/20210305/GIF/198147-94-3.gif)
198147-94-3
0 suppliers
inquiry

98777-34-5
18 suppliers
inquiry

85995-53-5
18 suppliers
$28.60/50MG

74-88-4
341 suppliers
$15.00/10g

98777-34-5
18 suppliers
inquiry