
Zileuton Impurity 2 synthesis
- Product Name:Zileuton Impurity 2
- CAS Number:1026256-93-8
- Molecular formula:C17H15NO3S
- Molecular Weight:313.37
Yield:1026256-93-8 85%
Reaction Conditions:
with boron trifluoride diethyl etherate at 5 - 30; for 2 h;
Steps:
5 Preparation of phenyl 1-(benzo[b]thiophen-2-yl)ethyl(hydroxyl) carbamate (V
The organic phase obtained in Example- 2, phenyl N-hydroxy carbamate (141.7 gm) was added and cooled to 5-10° C. Boron trifluoride dietherate (18.75 gm) was slowly added to the mixture at 25-30° C. for 2 hrs. The progress of the reaction was monitored by HPLC. After completion of the reaction, water (900 ml) was added to the reaction mass and stirred. The contents were cooled to 0-5° C., filtered, washed with cyclohexane and dried. % Yield: 85%
References:
US2016/376251,2016,A1 Location in patent:Paragraph 0114
![1-BENZO[B]THIOPHEN-2-YL-ETHANOL](/CAS/GIF/51868-95-2.gif)
51868-95-2
47 suppliers
$302.39/250MG
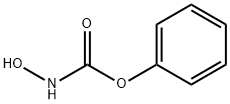
38064-07-2
23 suppliers
$76.00/1g

1026256-93-8
11 suppliers
inquiry
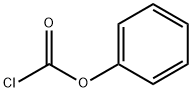
1885-14-9
332 suppliers
$10.00/1g

1026256-93-8
11 suppliers
inquiry
![2-Acetylbenzo[b]thiophene](/CAS/GIF/22720-75-8.gif)
22720-75-8
280 suppliers
$6.00/1g

1026256-93-8
11 suppliers
inquiry
![1-BENZO[B]THIOPHEN-2-YL-ETHANOL](/CAS/GIF/51868-95-2.gif)
51868-95-2
47 suppliers
$302.39/250MG

1026256-93-8
11 suppliers
inquiry
![Benzo[b]thiophene-2-methanol, α-methyl-, 2-acetate](/CAS/20211123/GIF/26929-23-7.gif)