
Zinc pyrithione synthesis
- Product Name:Zinc pyrithione
- CAS Number:13463-41-7
- Molecular formula:C10H8N2O2S2Zn
- Molecular Weight:317.7
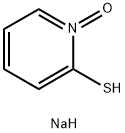
3811-73-2
431 suppliers
$18.00/25g
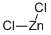
7646-85-7
570 suppliers
$10.00/25ml

13463-41-7
532 suppliers
$5.00/1g
Yield:13463-41-7 99.1%
Reaction Conditions:
in water at 70; for 1.16667 h;Product distribution / selectivity;
Steps:
1; 3; 5
Example 1: Preparation of Fine Powder of Zinc Pyrithione; 1000 g of sodium pyrithione, 20% aqueous solution (1.34 mmol of sodium pyrithione) was added to a 2L reactor equipped with a mechanical agitator and warmed to 70 °C with stirring. To the solution was added 190 g of 50 % zinc chloride (0.7 mmol of zinc chloride) over 10 minutes. After the completion of the addition, the solution was stirred at 70 °C for one hour, dehydrated and then washed with 500 g of distilled water. The solid thus obtained was dried with a heated-air drier at 70 °C to have a moisture content of less than 0.5 % and ground with a hammer mill to obtain a find powder of zinc pyrithione. The zinc pyrithione thus obtained was 212 g with a yield of 99.5 %. The purity checked by iodometry was 99.2 %. The particle size was measured with a particle size analyzer (Mastersiser 2000 supplied by Malvern Company), the results of which are presented in Table 1. The oil absorbency of the zinc pyrithione was 0.32 m/g as measured according to KS M ISO 787-5. The dispersability of the zinc pyrithione was 90 µm as measured according to KS M ISO 1524 and KS M ISO 8780-3, where a smaller value of the measurement indicates better dispersability.; Example 3: Preparation of Fine Powder of Zinc Pyrithione; 1000 g of sodium pyrithione, 20% aqueous solution (1.34 mmol of sodium pyrithione) was added to a 2L reactor equipped with a mechanical agitator and warmed to 70 °C with stirring. To the solution was added 190 g of 50 % zinc chloride (0.7 mmol of zinc chloride) over 10 minutes. After the completion of the addition, the solution was stirred at 70 °C for one hour, dehydrated and then washed with 500 g of distilled water. The solid thus obtained was dried with a heated-air drier at 70 °C to have a moisture content of less than 0.5 %. Two-thirds of the dry solid was ground with a hammer mill and mixed with one-third, which was not ground. The particle size was measured with a particle size analyzer (Mastersiser 2000 supplied by Malvern Company), the results of which are presented in Table 1. The zinc pyrithione thus obtained was 211 g with a yield of 99.1 %, and the purity checked by chemical analysis was 99.8 %. For the zinc pyrithione of which two-thirds was ground with a hammer mill and mixed with one-third that was not ground, oil absorbency and dispersability were 0.34 m /g and 100 µm, respectively, as measured in the same manner as described in Example 1.; Example 5: Preparation of Fine Powder of Zinc Pyrithione; 1000 g of sodium pyrithione, 20% aqueous solution (1.34 mmol of sodium pyrithione) was added to a 2L reactor equipped with a mechanical agitator and warmed to 70 °C with stirring. To the solution was added 190 g of 50 % zinc chloride (0.7 mmol of zinc chloride) over 10 minutes. After the completion of the addition, the solution was stirred at 70 °C for one hour, dehydrated and then washed with 500 g of distilled water. The solid thus obtained was filtered through a 50-mesh sieve, and the zinc pyrithione passed through the 50-mesh sieve was analyzed in regard to particle size with a particle size analyzer (Mastersiser 2000 supplied by Malvern Company). The results are presented in Table 1. The zinc pyrithione thus obtained was 212 g with a yield of 99.5 %, and the purity checked by chemical analysis was 98.8 %. For the zinc pyrithione, oil absorbency and dispersability were 0.35 m/g and 90 µm, respectively, as measured in the same manner as described in Example 1.
References:
KOLON INDUSTRIES, INC. EP1695963, 2006, A1 Location in patent:Page/Page column 4-5
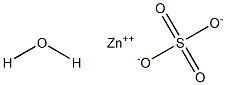
100685-55-0
0 suppliers
inquiry

3811-73-2
431 suppliers
$18.00/25g

13463-41-7
532 suppliers
$5.00/1g
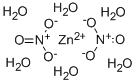
10196-18-6
0 suppliers
$14.00/100g

3811-73-2
431 suppliers
$18.00/25g

13463-41-7
532 suppliers
$5.00/1g