| Identification | More | [Name]
Trifluridine | [CAS]
70-00-8 | [Synonyms]
2'-DEOXY-5-TRIFLUOROMETHYLURIDINE
5-TRIFLUOROMETHYL-2'-DEOXYURIDINE
ALPHA,ALPHA,ALPHA-TRIFLUOROTHYMIDINE
F3TDR
TFT
TRIFLUORO-D-THYMIDINE
TRIFLUOROTHYMIDINE
TRIFLUOROTHYMINE DEOXYRIBOSIDE
TRIFLURIDINE
2,4(1h,3h)-pyrimidinedione,1-(2-deoxy-beta-d-ribofuranosyl)-5-(trifluorometh
2’-deoxy-5-(trifluoromethyl)-uridin
5-(trifluoromethyl)deoxyuridine
5-trifluoro-2’-deoxythymidine
alpha,alpha,alpha-trifluoro-thymidin
f3dthd
f3t
nsc75520
tfdu
trifluoromethyldeoxyuridine
viroptic | [EINECS(EC#)]
200-722-8 | [Molecular Formula]
C10H11F3N2O5 | [MDL Number]
MFCD00006534 | [Molecular Weight]
296.2 | [MOL File]
70-00-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
190-193 °C (lit.) | [density ]
1.4365 (estimate) | [refractive index ]
50 ° (C=1, H2O) | [storage temp. ]
−20°C
| [solubility ]
Soluble in DMSO (up to 25 mg/ml) or in Water (up to 14 mg/ml) | [form ]
solid | [pka]
pKa 7.85 (Uncertain) | [color ]
Off-white | [Merck ]
9687 | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 1 month. | [CAS DataBase Reference]
70-00-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
XP2087500
| [Hazard Note ]
Keep Cold | [HS Code ]
29349990 | [Hazardous Substances Data]
70-00-8(Hazardous Substances Data) | [Toxicity]
LD50 intraperitoneal in mouse: 1931mg/kg |
| Hazard Information | Back Directory | [Description]
Trifluorothymidine (70-00-8) is an analog of thymidine which inhibits thymidylate synthase possesses antiviral and anticancer activity.1,2 ?After phosphorylation by thymidine kinase, it is incorporated into DNA where it induces DNA-damage and interferes with repair enzymes.3 Enhances frame shift insertion and deletion in CRISPR genome editing in pluripotent stem cells.4 | [Chemical Properties]
White to Off-White Solid | [Originator]
Trifluorothymidine ,Mann,W. Germany,1975 | [Uses]
anti-herpesvirus antiviral agent | [Uses]
sunscreen | [Uses]
Trifluridine is used as antiviral agent in ophthalmie preparations. | [Definition]
ChEBI: A pyrimidine 2'-deoxyribonucleoside compound having 5-trifluoromethyluracil as the nucleobase. An antiviral drug used mainly in the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis. | [Indications]
Trifluridine (Viroptic) is a fluorinated pyrimidine nucleoside
that has in vitro activity against HSV-1 and HSV-
2, vaccinia, and to a lesser extent, some adenoviruses.
Activation of trifluridine requires its conversion to the
5 monophosphate form by cellular enzymes.Trifluridine
monophosphate inhibits the conversion of deoxyuridine
monophosphate (dUMP) to deoxythymidine
monophosphate (dTMP) by thymidylate synthetase. In
addition, it competes with deoxythymidine triphosphate
(dTTP) for incorporation by both viral and cellular
DNA polymerases. Trifluridine-resistant mutants have
been found to have alterations in thymidylate synthetase
specificity. | [Manufacturing Process]
A suspension of 4.00g (6.75 mmol) of 3',5'-bis-O-(p-nitrobenzoyl)-2'-deoxy-5-
(trifluoromethyl)uridine in 250 ml of methanol was treated with 10 ml of
diisopropylamine and refluxed until it had dissolved (about 18 minutes), and
the solution was concentrated. The dry residue was partitioned between 50 ml
of chloroform and 50 ml of water. The chloroform layer was washed with 20
ml of water, and the combined aqueous layers were concentrated. A low
ultraviolet extinction (ε 7200 and 262 μm; pH 1) and the presence of
isopropyl signals in the NMR spectrum (two singlets at γ8.73 and 8.85)
indicated the dry residue contained diisopropylamine, probably as a salt with
the relatively acidic heterocyclic N-H in 14.
A solution in 75 ml of water was treated with 8 ml (volume of resin) of Dowex
50 (H), prewashed with water and methanol. The resin was removed on a
filter and washed with 25 ml of methanol and 50 ml of water, The combined
filtrate was evaporated in vacuo to form 1.99 g of 2'-deoxy-4-
(trifluoromethyl)uridine (100%), melting point 171°C to 175°C. | [Brand name]
Viroptic (Monarch). | [Therapeutic Function]
Antiviral (ophthalmic) | [General Description]
Trifluridine, 5-trifluoromethyl-29-deoxyuridine (Viroptic),is a fluorinated pyrimidine nucleoside that demonstrates invitro inhibitory activity against HSV-1 and HSV-2, CMV,vaccinia, and some adenoviruses.Trifluridine possesses atrifluoromethyl group instead of an iodine atom at the 5-position of the pyrimidine ring.
synthetase, and the biologically generated triphosphatecompetitively inhibits thymidine triphosphate incorporationinto DNA by DNA polymerase. In addition, trifluridine inits triphosphate form is incorporated into viral and cellularDNA, creating fragile, poorly functioning DNA.Trifluridine is approved in the United States for the treatmentof primary keratoconjunctivitis and recurrent epithelialkeratitis caused by HSV types 1 and 2. Topical trifluridineshows some efficacy in patients with acyclovir-resistantHSV cutaneous infections. | [Pharmaceutical Applications]
Trifluorothymidine. A synthetic halogenated pyrimidine
nucleoside, first synthesized as an antitumor agent. It inhibits
enzymes of the DNA pathway and is incorporated into both
cellular and progeny viral DNA, causing faulty transcription of
late messenger RNA and the production of incompetent virion
protein. It does not require a viral thymidine kinase for monophosphorylation
and is far less selective and more toxic than
other analogs. It is active against HSV-1 and HSV-2, vaccinia
virus, CMV and possibly adenovirus. When applied as a 1%
ophthalmic solution, it rapidly enters the aqueous humor of
HSV-infected rabbits’ eyes but is cleared within 60–90 min.
It causes sister chromatid exchange – an indicator of mutagenicity
– at 0.5 mg/L in human lymphocytes and fibroblasts.
It is teratogenic to chick embryos when injected directly into
the yolk sac. Its principal adverse effects in humans following
systemic administration include leukopenia, anemia, fever
and hypocalcemia. Accordingly, it is restricted to topical ophthalmic
use in HSV ocular infections. The ophthalmic 1%
aqueous solution produces occasional punctate lesions; other
side effects are similar to those of idoxuridine but arise less
frequently. | [Biochem/physiol Actions]
Trifluorothymidine is a thymidine analog and is light sensitive. TFT serves as a thymidine kinase substrate to study enzyme specificity and kinetics. Incorporation of phosphorylated TFT into DNA induces damage, making it useful for DNA repair studies. TFT may also be used in the inhibition of thymidylate synthase and in screening mutant thymidine kinase gene.. It elicits antitumor activity in gastrointestinal (GI) cancers and has therapeutic potential to treat herpetic keratitis. | [Mechanism of action]
Trifluorothymidine is a fluorinated pyridine nucleoside structurally related to idoxuridine. It has been
approved by the U.S. FDA and is a potent, specific inhibitor of replication of HSV-1 in vitro. Its
mechanism of action is similar to that of idoxuridine. Like other antiherpes drugs, it is first
phos-phorylated by thymidine kinase to mono-, di-, and triphosphate forms, which are then incorporated
into viral DNA in place of thymidine to stop the formation of late virus mRNA and subsequent synthesis of
the virion proteins. | [Pharmacokinetics]
Trifluorothymidine, when given IV, shows a plasma half-life of 18 minutes and
is excreted in the urine either unchanged or as the inactive metabolite 5-carboxyuracil. | [Clinical Use]
Trifluridine is administered as a topical ophthalmic solution
for the treatment of primary keratoconjunctivitis
and recurrent keratitis due to HSV-1 or HSV-2. It is not
effective in the prophylaxis of these infections; however,
it is effective in treating patients who were unresponsive
or intolerant to topical idoxuridine or vidarabine. | [Side effects]
The most frequent adverse reactions to trifluridine administration
are transient burning or stinging and
palpebral edema. Other adverse reactions include superficial
punctate keratopathy, epithelial keratopathy,
hypersensitivity, stromal edema, irritation, keratitis
sicca, hyperemia, and increased intraocular pressure.
Trifluridine is mutagenic in vitro and carcinogenic
and teratogenic when administered subcutaneously to
animals. Topical trifluridine was not teratogenic in animal
studies. Because it is applied topically in humans,
the likelihood of systemic effects is low. | [Synthesis]
Trifluridine, 5-trifluoromethyl-1-(2-deoxyribofuranosyl)pyrimidin-2,
4-(1H.3H)-dione (36.1.22), is synthesized from 5-trifluoromethyluracil. This is synthesized
by the following scheme. It begins with trifluoroacetone, from which the oxynitrile (36.1.16)
is synthesized. Acetylation of this product gives the corresponding trifluoroacetate (36.1.16).
Pyrrolysis of this compound gives trifluoromethylacrylonitrile (36.1.17). Adding to this dry
hydrogen bromide in methanol solution in a process of which methanolysis of the nitrile
group takes place the bromide 36.1.19 is obtained, which upon acidic hydrolysis undergoes
heterocyclization to the dihydropyrimidine 36.1.20. Brominating of the obtained dihydropy�rimidine with molecular bromine and subsequent dehydrobromination of the resulting prod�uct 36.1.21 on heating in dimethylformamide gives 5-trifluoromethyluracil (36.1.22). This is
reacted with 2-deoxy-D-ribos-1-phosphate using the nucleoside phosphorylase enzyme, or
by treating it with hyxamethyldisylazane and then with trichloromethylsilane to make 2,4-
trimethylsilyloxy-5-trifluoromethyl pyrimidine (36.1.23).
Hexamethyldisilazane, which
itself does not form trimethylsilyl ethers, is used because using a combination of two
reagents leads to optimal yield of trimethylsilyl ethers. Reacting the resulting pyrimidine derivative with 3,5-bis-(4-nitrobenzoate)-2-deoxyribofuranosyl chloride in the presence of
mercury (II) acetate makes the corresponding ditrimethylsilyloxy nucleoside, which when
treated with an aqueous solution of potassium iodide to remove the protecting groups. The
resulting product undergoes preliminary purification by chromatography, and then is treated
with a methanol solution of diisopropylamine to remove the 4-nitrobenzoyl protection from
the furanosyl part, giving the desired trifluridine.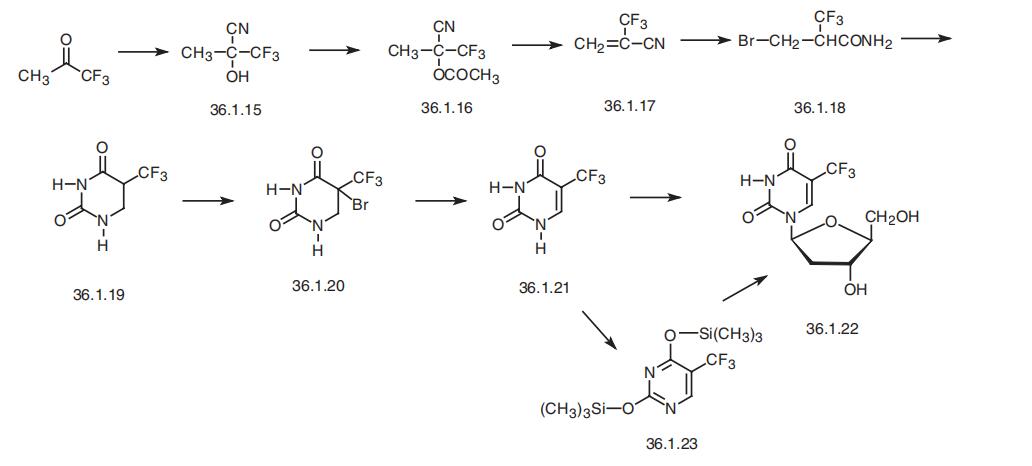
| [Veterinary Drugs and Treatments]
Trifluridine (trifluorothymidine; Viroptic?) is a pyrimidine nucleoside
analog. It is structurally related to 2-deoxythymidine, the natural
precursor of DNA synthesis. Trifluridine is poorly absorbed by
the cornea and is virostatic. Viroptic? interrupts viral replication
by substituting “nonsense” pyrimidine analogues. For this reason, a
competent surface immunity is necessary to resolve ocular disease,
with or without antiviral therapy. A recent in vitro study in which
several strains of feline herpes virus were collected from the United
States and were used to infect kidney epithelial cells showed that trifluridine
was more effective at lower concentrations compared with
several other agents. For this reason, trifluridine was the first choice
drug employed in the treatment of feline herpes virus ocular disease
for many years. Because of the topical toxicity associated with
use of trifluridine in cats, its popularity has diminished greatly. In
many milder cases, the irritation associated with topical trifluridine
is more intense then the inflammation induced by viral infection.
Antiviral agents have also been used in the treatment of superficial
punctate keratitis in the horse, thought to be associated with equine
herpes virus-2 (EHV-2) infection of the cornea. | [References]
1) Bijnsdorp et al. (2010), Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells; Int. J. Cancer, 126 2457
2) Temmink et al. (2010), Trifluorothymidine resistance is associated with decreased thymidine kinase and equilibrative nucleoside transporter expression or increased secretory phospholipase A2; Mol. Cancer Ther., 9 1047
3) Suzuki et al. (2011), Mode of action of trifluorothymidine (TFT) against DNA replication and repair enzymes; Int. J. Oncol., 39 263
4) Yu et al. (2015), Small molecules enhance CRISPR genome editing in pluripotent stem cells; Cell Stem Cell, 16 142 |
|
|