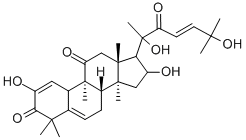
CUCURBITACIN I
- CAS No.
- 2222-07-3
- Chemical Name:
- CUCURBITACIN I
- Synonyms
- JSI-124;Ibamarin;ELATERIN B;NSC 521777;ELATERICIN B;CUCURBITACIN I;5-tetrahydroxy-;cucurbitacine(i);CUCURBITACIN I(SH);CUCURBITACIN I hplc
- CBNumber:
- CB6480840
- Molecular Formula:
- C30H42O7
- Molecular Weight:
- 514.65
- MDL Number:
- MFCD09971044
- MOL File:
- 2222-07-3.mol
- MSDS File:
- SDS
| Melting point | 148-150°C |
|---|---|
| Boiling point | 698.3±55.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | ≥22.45 mg/mL in DMSO; insoluble in EtOH; ≥51.2 mg/mL in H2O with ultrasonic |
| pka | 8.51±0.70(Predicted) |
| form | solid |
| color | white to off-white |
| LogP | 2.330 (est) |
| FDA UNII | SHQ47990PH |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P330+P331+P310 | |||||||||
| Hazard Codes | Xi,T+ | |||||||||
| Risk Statements | 25-28 | |||||||||
| Safety Statements | 1-22-45-36/37-28 | |||||||||
| RIDADR | UN 2811 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RC6200000 | |||||||||
| Toxicity | LD50 oral in mouse: 5mg/kg | |||||||||
| NFPA 704 |
|
CUCURBITACIN I price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHL89464 | Cucurbitacin I phyproof? Reference Substance | 2222-07-3 | 10MG | $598 | 2024-03-01 | Buy |
| Sigma-Aldrich | C4493 | Cucurbitacin I hydrate ≥95% (HPLC), solid | 2222-07-3 | 1mg | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | 238590 | Cucurbitacin I, Cucumis sativus L. A cell-permeable and irreversible bitter triterpenoid compound (NCI identifier: NSC 521777) that displays anti-proliferative and antitumor properties both in vitro and in vivo. | 2222-07-3 | 1MG | $289 | 2024-03-01 | Buy |
| Cayman Chemical | 14747 | Cucurbitacin I ≥98% | 2222-07-3 | 1mg | $96 | 2024-03-01 | Buy |
| Cayman Chemical | 14747 | Cucurbitacin I ≥98% | 2222-07-3 | 5mg | $238 | 2024-03-01 | Buy |
CUCURBITACIN I Chemical Properties,Uses,Production
Uses
Cucurbitacin I can be useful in the study of edible vitalmelon fruit extract and adipogenesis.
Definition
ChEBI: A cucurbitacin that is 9,10,14-trimethyl-4,9-cyclo-9,10-secocholesta-2,5,23-triene substituted by hydroxy groups at positions 2, 16, 20 and 25 and oxo groups at positions 1, 11 and 22.
Biological Activity
Selective inhibitor of STAT3/JAK2 signaling. Inhibits the activation of STAT3 and JAK2 and displays no activity on Src, Akt, ERK and JNK. Suppresses phosphotyrosine levels of STAT3, inhibits STAT3 DNA binding and STAT3-mediated gene expression. Induces apoptosis in cell lines expressing constitutively active tyrosine-phosphorylated STAT3.
Biochem/physiol Actions
Cucurbitacin I (JSI-124) is a novel selective inhibitor of the janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway with anti-proliferative and anti-tumor properties.
storage
Store at -20°C
References
blaskovich ma, sun j, cantor a et al. discovery of jsi-124 (cucurbitacin i), a selective janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice.cancer res. 2003 mar 15;63(6):1270-9.yu h, lee h, herrmann a et al. revisiting stat3 signalling in cancer: new and unexpected biological functions.nat rev cancer. 2014 nov;14(11):736-46. doi: 10.1038/nrc3818.song j, liu h, li z et al. cucurbitacin i inhibits cell migration and invasion and enhances chemosensitivity in colon cancer. oncol rep. 2015 apr;33(4):1867-71. qi j, xia g, huang cr et al. jsi-124 (cucurbitacin i) inhibits tumor angiogenesis of human breast cancer through reduction of stat3 phosphorylation. am j chin med. 2015;43(2):337-47.kim hj, kim jk et al. antiangiogenic effects of cucurbitacin-i. arch pharm res. 2015 feb;38(2):290-8. yuan g, yan sf, xue h et al. cucurbitacin i induces protective autophagy in glioblastoma in vitro and in vivo. j biol chem. 2014 apr 11;289(15):10607-19.
CUCURBITACIN I Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Aktin Chemicals, Inc. | 028-85159085 | info@aktinchem.com | CHINA | 1025 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Jiaozuo Furuitang Pharmaceutical Co., Ltd. | jzfrtcw@163.com | China | 8 | 58 |
View Lastest Price from CUCURBITACIN I manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-07-06 | CUCURBITACIN I
2222-07-3
|
US $1.00 / KG | 1G | 98% | 100KG | Career Henan Chemical Co |
-

- CUCURBITACIN I
2222-07-3
- US $1.00 / KG
- 98%
- Career Henan Chemical Co