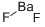불가화 바륨 C화학적 특성, 용도, 생산
개요
Barium fluoride appears as colourless, odourless crystals/white powder and is slightly
soluble in water but soluble in ethanol and methanol. When heated, barium fluoride
decomposes and emits toxic fumes of fluorine and barium. Barium fluoride is used as
a preopacifying agent and in enamel and glazing frits production. Its other use is in the
production of welding agents (an additive to some fluxes, a component of coatings for
welding rods and in welding powders). It is also used in metallurgy as a molten bath for
refining aluminium.
화학적 성질
Barium fluoride, BaF2, is a white crystal or powder.Under the microscope crystals may be clear and colorless. Barium fluoride is used commercially in combination with other fluorides for arc welding (qv) electrode fluxes.
물리적 성질
Barium fluoride (BaF2), also known as barium(II)
fluoride, is a solid which can also be a transparent
crystal. Its molecular weight is 175.34 g/mol. It occurs
in nature as the mineral “Frankdicksonite”. It occurs as
a white powder with a melting point of 1290 and a boiling point of 2259°C. It is slightly soluble in water
(0.159 at 0.0°C and 0.162 g/100 ml of water at 20°C). The solid generally assumes the fluorite structure, but
when subjected to high pressure, changes to the PbCl2
structure. In the vapor phase, the BaF2 molecule is
nonlinear with an F–Ba–F angle of approximately 108°C.
용도
Barium Fluoride is used in ceramic flux, carbon brushes for electrical equipment, glass making, in the manufacture of other fluorides, crystals for spectroscopy, electronics and dry-film lubricants. Barium Fluoride is used in spectroscopic components. Barium Fluoride is often suitable for applications in the passive IR band (8 to 14 ?m) and is often used as a viewport window for thermography.
Safety Profile
A poison by ingestion
and intraperitoneal routes. Moderately toxic
by subcutaneous route. An experimental
teratogen. See also FLUORIDES and
BARIUM COMPOUNDS (soluble). When
heated to decomposition it emits toxic
fumes of F-.
Purification Methods
Wash it well with distilled H2O and dry it in a vacuum. Its solubility in H2O is 1.6g (10o), 1.6g (20o) and 1.62g (30o) per L, and is soluble in mineral acids and aqueous NH4Cl. It may be stored in glass bottles. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 234 1963.]
Structure and conformation
The space lattice of Barium fluoride (BaF2) belongs to the cubic system, and its calcium fluoride structure has a lattice constant of a=0.6187 nm and Ba–F=0.268 nm.
불가화 바륨 준비 용품 및 원자재
원자재
준비 용품