DL-만딜오리트릴 C화학적 특성, 용도, 생산
화학적 성질
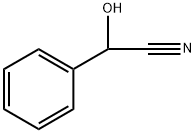
reddish-brown liquid. D,L-Mandelonitrile [532-28-5], benzaldehyde cyanohydrin, a-cyano-a-hydroxymethylbenzene, C6H5 – CH(OH) – CN, Mr 133.15, mp-10℃, d204 1.1165 – 1.120, is miscible with ethanol and diethyl ether, immiscible with water. Mandelonitrile occurs naturally as the bglycoside of gentiobiose (amygdalin). It decomposes on heating into benzaldehyde and hydrogen cyanide. It is produced from benzaldehyde and alkali cyanide in the presence of mineral acid. Mandelonitrile is used as an intermediate in the production of mandelic acid.
출처
Mandelonitrile, is a yellow, oily liquid, insoluble in water, but soluble in alcohol and diethyl ether. Mandelonitrile is a component of the glycoside amygdalin, a precursor of laetrile found in the leaves and seeds on most Prunus species (plum, peach, apricot, etc). In 1832, mandelonitrile was the first cyanohydrin to be synthesized. It is commercially prepared from benzaldehyde and hydrogen cyanide. Mandelonitrile is used by certain insects (tiger beetles, an African millipede) as a defense fluid. After expelling the fluid an enzyme catalyzes the conversion of mandelonitrile to benzaldehyde and HCN, which is usually fatal to the insect’s enemy.
용도
Mandelonitrile has been used to extract mandeloamide by the nitrilase variants.
정의
ChEBI: Mandelonitrile is a cyanohydrin that is phenylacetonitrile in which one of the methylene hydrogens is replaced by a hydroxy group.
일반 설명
Reddish-brown to dark red-brown liquid.
공기와 물의 반응
Mandelonitrile is sensitive to moisture. . Insoluble in water.
반응 프로필
Nitriles, such as Mandelonitrile, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Mandelonitrile is combustible.
Safety Profile
Poison by intravenous
and subcutaneous routes. Mutation data
reported. A severe eye irritant. When heated
to decomposition it emits toxic fumes of
NOx and CN-. See also NITRILES.
Toxicology
Mandelonitrile is rather toxic with an LD50 value (rat, oral) of 116 mg/kg. This high toxicity may be due to the equilibrium of the cyanohydrin with benzaldehyde and hydrogen cyanide.
DL-만딜오리트릴 준비 용품 및 원자재
원자재
준비 용품