- Plerixafor
-

- $921.60 / 10g/Bag
-
2023-11-13
- CAS:110078-46-1
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 5KG
- Plerixafor
-

- $50.00 / 1kg
-
2023-08-22
- CAS:110078-46-1
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10000tons
- Plerixafor
-

- $10.00 / 1kg
-
2023-07-31
- CAS:110078-46-1
- Min. Order: 20kg
- Purity: 99.95%
- Supply Ability: 500tons
|
| Product Name: | PLERIXAFOR | | Synonyms: | 1,1'-[1,4-Phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetradecane;1,4,8,11-Tetraazacyclotetradecane, 1,1'-(1,4-phenylenebis(methylene))bis-;Mozobil;Sdz sid 791;Unii-S915p5499n;1-[[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclotetradecane;Plerixafor(AMD3100);1-{[4-(1,4,8,11-tetraazacyclotetradecan-1-ylMethyl)phenyl]Methyl}-1,4,8,11-tetraazacyclotetradecane | | CAS: | 110078-46-1 | | MF: | C28H54N8 | | MW: | 502.78 | | EINECS: | 1592732-453-0 | | Product Categories: | Aromatics;Heterocycles;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;API | | Mol File: | 110078-46-1.mol | 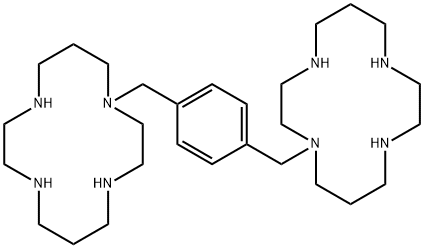 |
| | PLERIXAFOR Chemical Properties |
| Melting point | 122-125°C | | Boiling point | 657.5±55.0 °C(Predicted) | | density | 0.962 | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | Methanol (Slightly), Water (Slightly) | | form | Solid | | pka | 10.60±0.20(Predicted) | | color | White to Pale Beige | | Stability: | Hygroscopic | | CAS DataBase Reference | 110078-46-1 |
| | PLERIXAFOR Usage And Synthesis |
| Description | Plerixafor (AMD3100) is a chemokine receptor antagonist for CXCR4 and CXCL12-mediated chemotaxis with IC50 of 44 nM and 5.7 nM in cell-free assays, respectively. | | In vitro | Plerixafor inhibits CXCL12-mediated chemotaxis with a potency lightly better than its affinity for CXCR4. Plerixafor also antagonizes SDF-1/CXCL12 ligand binding with an IC50 of 651 nM. Plerixafor inhibits SDF-1 mediated GTP-binding, SDF-1 mediated calcium flux and SDF-1 stimulated chemotaxis with IC50 of 27 nM, 572 nM and 51 nM, respectively. Plerixafor does not inhibit calcium flux against cells expressing CXCR3, CCR1, CCR2b, CCR4, CCR5 or CCR7 when stimulated with their cognate ligands, nor does Plerixafor inhibit receptor binding of LTB4. Plerixafor does not, on its own, induce a calcium flux in the CCRF–CEM cells, which express multiple GPCRs including CXCR4, CCR4 and CCR7. | | In vivo | A single topical application of Plerixafor promotes wound healing in diabetic mice by increasing cytokine production, mobilizing bone marrow EPCs, and enhancing the activity of fibroblasts and monocytes/macrophages, thereby increasing both angiogenesis and vasculogenesis. Cohorts of mice are administered with PBS, IGF1, PDGF, SCF, or VEGF for five consecutive days and Plerixafor on the 5th day. The number and size of the colonies are highest in IGF1 plus Plerixafor injected mice compared to PDGF, SCF and VEGF treated groups, in combination with Plerixafor. | | Description | Autologous hematopoietic stem cell (HSC) transplantation, a standard
treatment for hematological malignancies, such as non-Hodgkin’s lymphoma or multiple myeloma, involves collection of HSCs from the
patient, chemo- or radiotherapy of the patient to eliminate malignant cells,
and retransplantation of the stored HSCs.
Plerixafor is a potent antagonist of the CXCR4 chemokine receptor and a first-in-class drug for stem cell mobilization. CXCR4
is specific for stromal-derived-factor-1 (SDF-1), a molecule endowed with
potent chemotactic activity forlymphocytes. Because the interaction between
SDF-1 and CXCR4 plays an important role in holding HSCs in the bone
marrow, drugs that block the activity of CXCR4 receptor are capable of
mobilizing HSCs into the bloodstream. In vitro, plerixafor potently blocks
SDF-1 binding of CXCR4 and inhibits SDF-1-induced calcium flux
(IC50 = 0.01-0.13 mg/mL) and chemotaxis (IC50 = 0.13 mg/mL) in several
different cell types.
Plerixafor, in combination with G-CSF, is specifically
indicated to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma. | | Chemical Properties | White Solid | | Originator | AnorMED (Canada) | | Uses | Plerixafor is a hematopoietic stem cell (HSC) mobilizer that inhibits the CXCR4 chemokine receptor and blocks binding of its ligand, stromal cell-derived factor-1-α (SDF-1-α). This agent was approved on Dec. 15, 2008, as treatment in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin''s lymphoma (NHL) and multiple myeloma (MM). Selective CXCR4 antagonist. | | Uses | Plerixafor is a chemokine receptor antagonist for CXCR4 and CXCL12-mediated chemotaxis with IC50 of 44 nM and 5.7 nM, respectively | | Definition | ChEBI: Plerixafor is an azamacrocycle consisting of two cyclam rings connected by a 1,4-phenylenebis(methylene) linker. It is a CXCR4 chemokine receptor antagonist and a hematopoietic stem cell mobilizer. It is used in combination with grulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the perpheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma. It has a role as an immunological adjuvant, an antineoplastic agent, an anti-HIV agent and a C-X-C chemokine receptor type 4 antagonist. It is an azamacrocycle, a crown amine, a secondary amino compound, an azacycloalkane, a tertiary amino compound and a member of benzenes. It is functionally related to a 1,4,8,11-tetraazacyclotetradecane. | | Brand name | Mozobil | | Clinical Use | Chemokine receptor antagonist:
To enhance mobilisation of haematopoietic stem
cells to the peripheral blood for collection and
subsequent autologous transplantation in patients
with lymphoma and multiple myeloma whose cells
mobilise poorly | | Side effects | The most common adverse reactions (occurring in more than 10% of patients) observed during plerixafor use include diarrhea, nausea, fatigue, injection-site reactions, headache, arthralgia, dizziness, and vomiting. | | Synthesis | Plerixafor can be synthesized by several related procedures starting from tetraazacyclotetradecane, the macrocyclic tetraamine cyclam. The general synthetic strategy entails the masking of three of the four ring nitrogens, followed by alkylation with para-xylylene dibromide, and subsequent removal of the masking groups. In one approach, tetraazacyclotetradecane is protected as the phosphorotriamide derivative by reaction with tris (dimethylamino)phosphine followed by oxidation with carbon tetrachloride and sodium hydroxide. After condensation with xylylene dibromide, the dimeric bis-phosphoramide intermediate is hydrolyzed to plerixafor by treatment with dilute hydrochloric acid solution. | | Metabolism | Not metabolised.
About 70% of a dose is eliminated in the urine within 24
hours. | | references | [1]zabel ba, wang y, lewén s, berahovich rd, penfold me, zhang p, powers j, summers bc, miao z, zhao b, jalili a, janowska-wieczorek a, jaen jc, schall tj. elucidation of cxcr7-mediated signaling events and inhibition of cxcr4-mediated tumor cell transendothelial migration by cxcr7 ligands. j immunol. 2009 sep 1;183(5):3204-11.
[2].li j, oupicky d. effect of biodegradability on cxcr4 antagonism, transfection efficacy and antimetastatic activity of polymeric plerixafor.biomaterials. 2014 jul;35(21):5572-9.
[3]. broxmeyer he. chemokines in hematopoiesis. curr opin hematol. 2008 jan;15(1):49-58.
[4]. devi s, wang y, chew wk, lima r, a-gonzález n, mattar cn, chong sz, schlitzer a, bakocevic n, chew s, keeble jl, goh cc, li jl, evrard m, malleret b, larbi a, renia l, haniffa m, tan sm, chan jk, balabanian k, nagasawa t, bachelerie f, hidalgo a, ginhoux f, kubes p, ng lg. neutrophil mobilization via plerixafor-mediated cxcr4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. j exp med. 2013 oct 21;210(11):2321-36.
[5]. mcdermott dh, liu q, velez d, lopez l, anaya-o'brien s, ulrick j, kwatemaa n, starling j, fleisher ta, priel da, merideth ma, giuntoli rl, evbuomwan mo, littel p, marquesen mm, hilligoss d, decastro r, grimes gj, hwang st, pittaluga s, calvo kr, stratton p, cowen ew, kuhns db, malech hl, murphy pm. a phase 1 |
| | PLERIXAFOR Preparation Products And Raw materials |
|