- Cadmium Acetate
-

- $0.00 / 1Kg
-
2024-04-10
- CAS:543-90-8
- Min. Order: 1Kg
- Purity: 99.99%
- Supply Ability: 20 tons
- Cadmium acetate
-

- $15.00 / 1KG
-
2021-07-02
- CAS:543-90-8
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Cadmium acetate
-

- $0.10 / 1KG
-
2020-01-05
- CAS:543-90-8
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 1000 tons
|
| | Cadmium acetate Basic information |
| Product Name: | Cadmium acetate | | Synonyms: | Cadmium Acetate, Anhydrous 99%;Cadmium(II) acetate anhydrous, 99.995%;Cadmium Acetate Anhydrous≥99.95%;Cadmium acetate anhydrous≥ 98% (Assay);Cadmium acetate anhydrous≥99.99%;ai3-01414;bis(acetoxy)cadmium;Nsc75795 | | CAS: | 543-90-8 | | MF: | C4H6CdO4 | | MW: | 230.5 | | EINECS: | 208-853-2 | | Product Categories: | metal acetate salt;Organic-metal salt | | Mol File: | 543-90-8.mol | 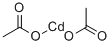 |
| | Cadmium acetate Chemical Properties |
| Provider | Language |
|
ALFA
| English |
| | Cadmium acetate Usage And Synthesis |
| Description | Cadmium acetate is a colourless crystal with a characteristic odour. It is not combustible,
but it decomposes on heating, producing toxic fumes of cadmium oxide. It is incompatible
with oxidising agents, metals, hydrogen azide, zinc, selenium, and tellurium. Occupational
exposure to cadmium and cadmium compounds occurs in workplaces mainly in the form
of airborne dust and fume. Occupations and workplaces include cadmium production and
refining, nickel–cadmium battery manufacture, cadmium pigment manufacture and formulation,
cadmium alloy production, mechanical plating, zinc smelting, soldering, and polyvinylchloride
compounding. Cadmium and compounds enter the body mainly by inhalation
and by ingestion. | | Chemical Properties | White crystalline powder | | Chemical Properties | Cadmium acetate is a colorless crystalline
solid; freezing/melting point 5 130�C. Hazard identification
(based on NFPA-704 M Rating System): Health 3, flammability 0, reactivity 0. Soluble in water | | Chemical Properties | Cadmium acetate is colorless crystal with a characteristic odor. It is not combustible, but it
decomposes on heating, producing toxic fumes of cadmium oxide. It is incompatible with oxidizing agents, metals, hydrogen azide, zinc, selenium, and tellurium. Occupational
exposure to cadmium and cadmium compounds occurs in workplaces mainly in the form
of airborne dust and fumes. Occupations and workplaces include cadmium production
and refi ning, nickel-cadmium battery manufacture, cadmium pigment manufacture and
formulation, cadmium alloy production, mechanical plating, zinc smelting, soldering, and
polyvinylchloride compounding. Cadmium and compounds enter the body mainly by
inhalation and by ingestion | | Physical properties | The anhydrous salt occurs as a colorless crystal while the dihydrate is a white crystalline solid; faint odor of acetic acid; density 2.34 g/cm3 (dihydrate2.01 g/cm3); melts at 255°C; dihydrate decomposes at 130°C; soluble in water and ethanol; pH of 0.2M aqueous solution 7.10. | | Uses | Cadmium acetate is used for glazing ceramics and pottery; in electroplating baths; in dyeing and printing textiles; and as an analytical reagent for sulfur, selenium, and tellurium. | | Uses | Cadmium(II) acetate can be used in the synthesis of cadmium oxide (CdO) thin films, which find usage in gas sensors, phototransistors, and diodes. It can also be used in the synthesis of cadmium sulfide (CdS) nanoparticles, which can be used in a variety of optoelectronic devices. | | Preparation | Cadmium acetate is prepared by treating cadmium oxide with acetic acid:
CdO + 2CH3COOH → (CH3COO)2Cd + H2O
Also, the compound may be prepared by treating cadmium nitrate with acetic anhydride. | | General Description | Odorless colorless solid. Sinks and mixes with water. | | Air & Water Reactions | Slowly oxidized by moist air to form cadmium oxide [Merck 11th ed. 1989]. Water soluble. | | Reactivity Profile | Salts, basic, such as Cadmium acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Special Hazards of Combustion Products: Toxic cadmium oxide fumes may form in fires [USCG, 1999]. | | Health Hazard | Inhalation causes coughing, sneezing, symptoms of lung damage. Ingestion produces severe toxic symptoms; both kidney and liver injuries may occur. Contact with dust causes eye irritation. | | Health Hazard | Exposures to cadmium acetate cause cough, skin redness, abdominal pain, nausea, vomiting, salivation, choking, dizziness, and diarrhea. On catching fi re, cadmium acetate gives
off irritating or toxic metal oxide fumes. Inhalation of dust produces perforation of the
nasal septum, loss of smell, irritation, headache, metallic taste, and cough. Prolonged
exposures to cadmium acetate may produce shortness of breath, chest pain, and fl u-like
symptoms, chills, weakness, fever, muscular pain, pulmonary edema, liver and kidney
damage and death. Cadmium acetate may have effects on the kidneys and bones, leading
to kidney impairment and osteoporosis (bone weakness), and liver damage. Accidental
ingestion or inhalation of cadmium acetate may be fatal to workers | | Fire Hazard | Special Hazards of Combustion Products: Toxic cadmium oxide fumes may form in fires. | | Safety Profile | Confirmed human
carcinogen. Poison by intraperitoneal route. An experimental teratogen. Other
experimental reproductive effects. Human
mutation data reported. When heated to
decomposition it emits toxic fumes of Cd.
See also CADMIUM COMPOUNDS. | | Potential Exposure | Cadmium acetate is a colorless crystalline
solid; freezing/melting point 5 130�C. Hazard identification
(based on NFPA-704 M Rating System): Health 3, flammability 0, reactivity 0. Soluble in water | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR if heartaction has stopped. Transfer promptly to a medical facility.When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Donot make an unconscious person vomit. Medical observationis recommended for 24-48 h after breathing overexposure,as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective inpoisoning from cadmium. | | storage | Color Code—Blue: Health Hazard: Store in asecure poison location. Prior to working with Cadmiumacetate you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from heat and incompatible materialslisted above. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045. | | Shipping | UN2570 Cadmium compounds, Hazard Class:
6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. | | Incompatibilities | Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water, and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, sulfides (releasing
heat, toxic, and possibly flammable gases), thiosulfates,
and dithionites (releasing hydrogen sulfate and oxides of
sulfur). Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides | | Waste Disposal | Precipitation as sulfide, drying and return to supplier. Incineration is not
recommended. | | Precautions | During use and handling of cadmium acetate, occupational workers should be careful.
Workers should use protective gloves and immediately remove contaminated clothing
and shoes. The workplace should provide an eye-wash fountain and quick-drench facilities. During use of cadmium acetate, workers should avoid heat, flame, ignition sources,
dust, and incompatibles. |
| | Cadmium acetate Preparation Products And Raw materials |
|