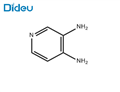
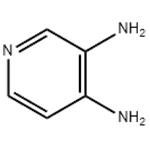
- 3,4-Diaminopyridine
-
- $1.10 / 1g
-
2024-04-17
- CAS:54-96-6
- Min. Order: 1g
- Purity: 99.0% min
- Supply Ability: 100 tons min
- 3,4-Diaminopyridine
-
- $34.00 / 1kg
-
2024-03-23
- CAS:54-96-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 3,4-Diaminopyridine
-

- $0.00 / 25KG
-
2023-07-05
- CAS:54-96-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| | 3,4-Diaminopyridine Chemical Properties |
| Melting point | 216-218 °C (lit.) | | Boiling point | 194.6°C (rough estimate) | | density | 1.1118 (rough estimate) | | refractive index | 1.5340 (estimate) | | Fp | 240 °C | | storage temp. | Store below +30°C. | | solubility | 30g/l | | pka | 9.17±0.12(Predicted) | | form | Crystalline Powder | | color | Brownish to brown-gray | | Water Solubility | 24 g/L (20 ºC) | | Merck | 14,2986 | | BRN | 110232 | | InChI | InChI=1S/C5H7N3/c6-4-1-2-8-3-5(4)7/h1-3H,7H2,(H2,6,8) | | InChIKey | OYTKINVCDFNREN-UHFFFAOYSA-N | | SMILES | C1=NC=CC(N)=C1N | | CAS DataBase Reference | 54-96-6(CAS DataBase Reference) |
| Hazard Codes | T+,T,Xi | | Risk Statements | 25-26-36/37/38 | | Safety Statements | 26-28-36/37/39-45-37/39-28A-36 | | RIDADR | UN 2811 6.1/PG 2 | | WGK Germany | 3 | | RTECS | US7600000 | | F | 10-23 | | Autoignition Temperature | 480 °C | | Hazard Note | Irritant | | HazardClass | 6.1 | | PackingGroup | I | | HS Code | 29333999 | | Toxicity | LD50 orally in Rabbit: 47 mg/kg LD50 dermal Rabbit > 200 mg/kg |
| | 3,4-Diaminopyridine Usage And Synthesis |
| Description | 3,4-diaminopyridine (3,4-DAP, amifampridine) is the leading treatment for Lambert–Eaton myasthenic syndrome (LEMS), an autoimmune disorder with impaired neuromuscular transmission, for which few effective medications are currently available. 3,4-DAP has been available as a therapy for LEMS in special treatment programmes for approximately 25 years. | | Uses | Amifampridine (3,4-Diaminopyridine) is a drug, predominantly in the treatment of a number of rare muscle diseases. 3,4-Diaminopyridine (3,4-DAP) is used in the treatment of Lambert-Eaton myasthenic syndrome (LEMS) and some congenital myasthenic syndromes (CMS). It is used to improve muscle strength. | | Mechanism of action | Amifampridine works by blocking potassium channel efflux in nerve terminals so that action potential duration is increased. Ca2+ channels can then be open for a longer time and allow greater acetylcholine release to stimulate muscle at the end plate. | | Pharmacokinetics | Administration of amifampridine to patients with LES in clinical trials resulted in improvement of the compound muscle action potential (CMAP), muscle function, and quantitative myasthenia gravis (QMG) score . One case of a slight prolongation of the QTc interval in male patient with LEMS and euthyroid Hashimoto’s disease treated with 90 mg of amifampridine in combination with 100 mg azathioprine was reported . In vitro, amifampridine was shown to modulate cardiac conduction and induce phasic contractions in different arteries from several species. In addition, it stimulated potassium-evoked dopamine and noradrenaline release in rat hippocampal slices and upregulate acetylcholine release in the brain. It may also potentiate adrenergic and cholinergic neuromuscular transmission in the gatrointestinal tract. In a single pharmacokinetic study, no effect was observed of amifampridine phosphate on cardiac repolarization as assessed using the QTc interval . There were no changes in heart rate, atrioventricular conduction or cardiac depolarization as measured by the heart rate, PR and QRS interval durations. | | Toxicty | The approximate oral LD50 was >25mg/kg in rats and 100 mg/kg in mice. The approximate intravenous LD50 was 25 mg/kg in both rats and mice. Peritoneal and subcutaneous LD50 in mice were 20 mg/kg and 35 mg/kg, respectively. There is limited clinical experienced with amifampridine overdose. The manifestations of acute drug overdose may include abdominal pain, and should be responded with discontinuation of treatment and initiation of supportive care with close monitoring of viral signs. There is no specific antidote known for amifampridine . | | Chemical Properties | brownish to brown-grey crystalline powder | | Uses | It is a potassium channel blocker in nerve terminals. It inhibits potassium channel efflux, increasing the duration of the action potential, which results in an increase in the duration of calcium channel opening and enhanced acetylcholine (ACh) release. Increased ACh availability at the motor end plate allows muscles to contract. | | Indications | 3,4-diaminopyridine has been used to treat Lambert Eaton myasthenia (LEM) for thirty years despite the lack of conclusive evidence of efficacy.
Lambert–Eaton myasthenic syndrome is characterized by muscle weakness, hyporeflexia, and autonomic dysfunction, which result from impaired release of acetylcholine from cholinergic nerve terminals. It is frequently associated with cancer, it is autoimmune-mediated, and treatment has been unsatisfactory.
The drug 3,4-diaminopyridine (3,4-DAP) increases neurotransmitter release and also the action potential (by blocking potassium conductance); these actions lead to a nonspecific excitatory effect on the cholinergic system, and provide benefit. It should be taken orally, 4-5 times per day. Adverse effects due to CNS excitation (insomnia, seizures) can occur. | | Definition | ChEBI: Amifampridine is an aminopyridine. | | Purification Methods | It crystallises from *benzene and is stored under N2 because it is deliquescent and absorbs CO2. [Beilstein 22/11 V 266.] |
| | 3,4-Diaminopyridine Preparation Products And Raw materials |
|