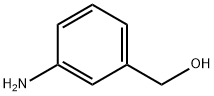
3-Aminobenzylalcohol synthesis
- Product Name:3-Aminobenzylalcohol
- CAS Number:1877-77-6
- Molecular formula:C7H9NO
- Molecular Weight:123.15
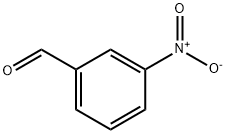
99-61-6
629 suppliers
$5.00/10g

1877-77-6
242 suppliers
$5.00/250mg
Yield:1877-77-6 97%
Reaction Conditions:
Stage #1:3-nitro-benzaldehyde with nickel boride in water at 20; for 0.0833333 h;Green chemistry;
Stage #2: with sodium tetrahydroborate in water at 20; for 0.133333 h;Green chemistry;
Steps:
A typical procedure for reduction of nitrobenzene to anilinewith NaBH4/additive Ni2B system
General procedure: In a round-bottomed flask (10 mL) equipped with a magneticstirrer, a mixture of nitrobenzene (0.123 g, 1 mmol)and H2O (2 mL) was prepared. Ni2B (0.006 g, 0.05 mmol) was then added and the mixture was stirred for 5 min.NaBH4 (0.095 g, 2.5 mmol) was also added and the resultingmixture was continued to stirring for 3 min at roomtemperature. TLC monitored the progress of the reaction(eluent, n-hexane/Et2O:5/3). After completion of the reaction,aqueous solution of KOH (2 %, 5 mL) was addedand the mixture was stirred for 10 min. The mixture wasextracted with EtOAc (3 × 8 mL) and then dried overanhydrous Na2SO4. Evaporation of the solvent affords thepure liquid aniline in 95 % yield (0.088 g, Table 2, entry 1).
References:
Zeynizadeh, Behzad;Zabihzadeh, Mehdi [Journal of the Iranian Chemical Society,2015,vol. 12,# 7,art. no. 585,p. 1221 - 1226]
619-25-0
213 suppliers
$8.00/5g

1877-77-6
242 suppliers
$5.00/250mg
99-05-8
500 suppliers
$14.00/5g

1877-77-6
242 suppliers
$5.00/250mg

1709-44-0
64 suppliers
inquiry

1877-77-6
242 suppliers
$5.00/250mg

24964-64-5
318 suppliers
$9.00/1g

1877-77-6
242 suppliers
$5.00/250mg