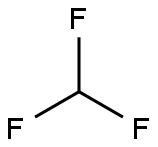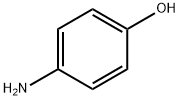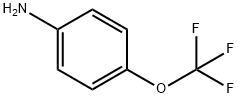
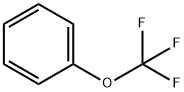
4-(Trifluoromethoxy)aniline synthesis
- Product Name:4-(Trifluoromethoxy)aniline
- CAS Number:461-82-5
- Molecular formula:C7H6F3NO
- Molecular Weight:177.12
456-55-3
300 suppliers
$6.00/10g

461-82-5
441 suppliers
$10.00/1g
Yield:461-82-5 98.2%
Reaction Conditions:
Stage #1:1-trifluoromethoxybenzene with sodium ferrate(VI);sodium bromide in dimethyl sulfoxide at 95; for 4 h;Inert atmosphere;
Stage #2: with sodium amide in dimethyl sulfoxide at 155; under 3040.2 Torr; for 10 h;Inert atmosphere;Solvent;Temperature;Reagent/catalyst;Pressure;
Steps:
1 Example 1 The method for synthesizing trifluoromethoxyaniline comprises the following steps:
1) Add 1 mol of trifluoromethoxybenzene and anhydrous DMSO to the reaction vessel under argon and vigorous stirring. Then add sodium ferrate and sodium bromide as auxiliary reaction mixture, heat to 95 ° C for 4 h, then add sodium amide, The temperature was raised to 155 ° C, the reaction pressure was raised to 4 atm, and the reaction was continued for 10 h; The ratio of trifluoromethoxybenzene to sodium amide is 1:4.5, The ratio of the amount of the trifluoromethoxybenzene to the auxiliary reaction mixture was 1:1.4. The ratio of sodium ferrate to sodium bromide is 1:1. 2) After cooling the system, pour it into 8 volumes of water. The extract was extracted with 4 times the original reaction volume of chloroform, and the extract was washed with water and dried over anhydrous sodium sulfate. Then concentrated to give the product in a molar yield of 98.2%, HPLC purity 97.7%
References:
Shandong Nong Pharmaceutical Xue Institute;Jinan Kehai Co., Ltd.;Fu Hongxin;Yang Chaohui;Wang Ling CN109134277, 2019, A Location in patent:Paragraph 0025-0049
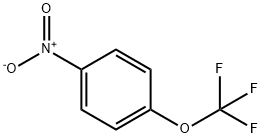
713-65-5
227 suppliers
$8.00/1g

461-82-5
441 suppliers
$10.00/1g
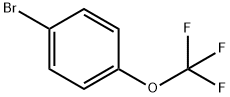
407-14-7
383 suppliers
$5.00/1g

461-82-5
441 suppliers
$10.00/1g

41419-59-4
71 suppliers
$32.00/1 g

461-82-5
441 suppliers
$10.00/1g