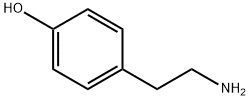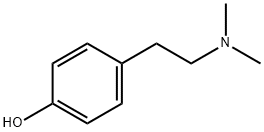
Hordenine synthesis
- Product Name:Hordenine
- CAS Number:539-15-1
- Molecular formula:C10H15NO
- Molecular Weight:165.23
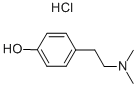
6027-23-2
214 suppliers
$45.00/100mg

539-15-1
304 suppliers
$11.00/5g
Yield:539-15-1 71.54%
Reaction Conditions:
with hydrogen bromide at 120 - 130;
Steps:
3 Example 3 Preparation of desvenlafaxine succinate impurity 6
Add 2.00 g (37 mmol) of 2-(4-methoxyphenyl)-N,N-dimethylethylamine hydrochloride and 80 ml (40%) of hydrobromic acid to the reaction flask and stir and heat to 120~130. °C reaction 3 to 5 hours.After the reaction is complete, cool down to 0~20°C and add 40% sodium hydroxide solution to adjust the pH to 9~11.Ethyl acetate 24ml was added and the mixture was separated, the aqueous phase was extracted with ethyl acetate 24ml, and the organic phases were combined.Add 12g of anhydrous sodium sulfate to dry and dehydrate, filter, collect the filtrate and concentrate to dryness under vacuum. Add 8ml of ethyl acetate to the residue and stir to warm up to 65~75°C.After dissolving, cool down to 5~15°C, incubate and crystallize, filter, and filter cake is dried under reduced pressure to obtain target compound.4-(2-(N,N-dimethylamino)ethyl)phenol (Impurity 6) 4.35 g, yield 71.54%, purity 98.64%.
References:
Chongqing Zhien Pharmaceutical Co., Ltd.;Deng Xianglin;Huang Minghui;Song Yuecheng;Wang Peng;Liao Xingting;Gou Juan;Zhong Qichang;Sun Yi;Mu Xiang;Zhao Weishu;Li Daming CN107935869, 2018, A Location in patent:Paragraph 0045
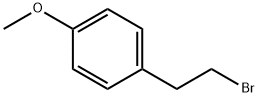
14425-64-0
58 suppliers
$22.00/250mg

124-40-3
499 suppliers
$18.00/100ml

539-15-1
304 suppliers
$11.00/5g

918344-20-4
21 suppliers
inquiry

124-40-3
499 suppliers
$18.00/100ml

539-15-1
304 suppliers
$11.00/5g
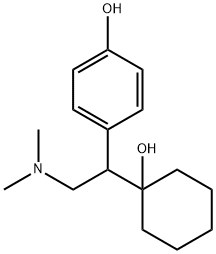
93413-62-8
233 suppliers
$5.00/5mg
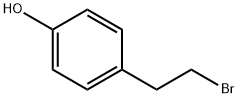
14140-15-9
83 suppliers
$8.00/250mg

124-40-3
499 suppliers
$18.00/100ml

539-15-1
304 suppliers
$11.00/5g