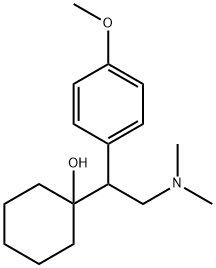
Venlafaxine synthesis
- Product Name:Venlafaxine
- CAS Number:93413-69-5
- Molecular formula:C17H27NO2
- Molecular Weight:277.4
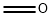
50-00-0
843 suppliers
$10.00/25g

64-18-6
1057 suppliers
$22.12/250ML
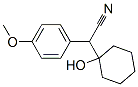
93413-76-4
241 suppliers
$22.00/100mg

93413-69-5
249 suppliers
$10.00/1mg
Yield:93413-69-5 78%
Reaction Conditions:
with hydrogen;platinum on carbon in methanol;water at 40 - 50; under 7355.72 - 14711.4 Torr; for 10 - 24 h;Product distribution / selectivity;
Steps:
6
An autoclave was charged with methanol (200 ml), l-[Cyano-(4- methoxyphenyl)methyl]-cyclohexanol (II) (25 g, 0.1019 mole) , palladium on charcoal (10%, 50% wet) (25 g) , hydrochloric acid (1-3 mole). While supplying hydrogen gas at 0- 20 kg/cm2, the mixture was slowly heated to 4O0C and then heated to 40-500C for about 7- 12 hours with hydrogen pressure 15-20 kg/cm2. After completion of the reaction, the mass EPO
References:
TEVA PHARMACEUTICAL INDUSTRIES LTD.;TEVA PHARMACEUTICALS USA, INC. WO2007/47972, 2007, A2 Location in patent:Page/Page column 19

93413-76-4
241 suppliers
$22.00/100mg
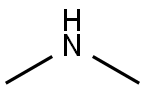
124-40-3
500 suppliers
$18.00/100ml

93413-69-5
249 suppliers
$10.00/1mg
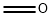
50-00-0
843 suppliers
$10.00/25g

93413-76-4
241 suppliers
$22.00/100mg

93413-69-5
249 suppliers
$10.00/1mg
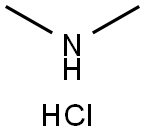
506-59-2
528 suppliers
$5.00/5 g

93413-76-4
241 suppliers
$22.00/100mg

93413-69-5
249 suppliers
$10.00/1mg
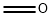
50-00-0
843 suppliers
$10.00/25g

93413-69-5
249 suppliers
$10.00/1mg