
N-Ethyl-2-methylbenzimidazole synthesis
- Product Name:N-Ethyl-2-methylbenzimidazole
- CAS Number:5805-76-5
- Molecular formula:C10H12N2
- Molecular Weight:160.22

75-07-0
410 suppliers
$14.00/5mL
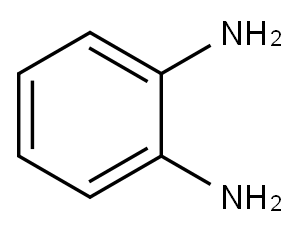
95-54-5
488 suppliers
$9.00/1g
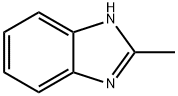
615-15-6
315 suppliers
$9.00/1g

5805-76-5
35 suppliers
inquiry
Yield:5805-76-5 98% ,615-15-6 2%
Reaction Conditions:
with erbium(III) triflate at 80; for 0.0333333 h;Green chemistry;
Steps:
General experimental procedure for the synthesis of 1,2-disubstituted benzimidazoles.
General procedure: Phenylenediamine (0.5 mmol, 0.054 g) and Er(OTf)3(0.05 mmol, 0.031 g) were added to the aldehyde (1 mmol). Solid aldehydes were dissolved in 2 mL of ethanol. Liquid aldehydes were used without solvent. The reaction mixture was stirred at 80 °C for 2 minutes. The crude product was extracted with dichloromethane and water. The organic extract was analyzed by GC-MS, and the products were isolated by radial chromatography, eluting with hexane/ethyl acetate (70:30). Solid products were recrystallized from ethanol. The use of green solvents such as cyclopentyl methyl ether or methyl tert-butyl ether in the work-up is possible, but slightly decreased the product yields from 91% to 88 and 85%, respectively.
References:
Herrera Cano, Natividad;Uranga, Jorge G.;Nardi, Mónica;Procopio, Antonio;Wunderlin, Daniel A.;Santiago, Ana N. [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 2410 - 2419] Location in patent:supporting information

444995-69-1
0 suppliers
inquiry

5805-76-5
35 suppliers
inquiry

19056-34-9
22 suppliers
$45.00/5mg

5805-76-5
35 suppliers
inquiry

24340-87-2
15 suppliers
inquiry

5805-76-5
35 suppliers
inquiry